DTR Medical (Llansamlet, UK), a manufacturer of single-use surgical instruments, has specified Sorona, a renewably sourced polymer from DuPont (Le Grand-Saconnex, Switzerland), for the fabrication of six components in its Cervical Rotating Biopsy Punch. DuPont is exhibiting the device at Fakuma this week in Friedrichshafen, Germany.
October 16, 2014
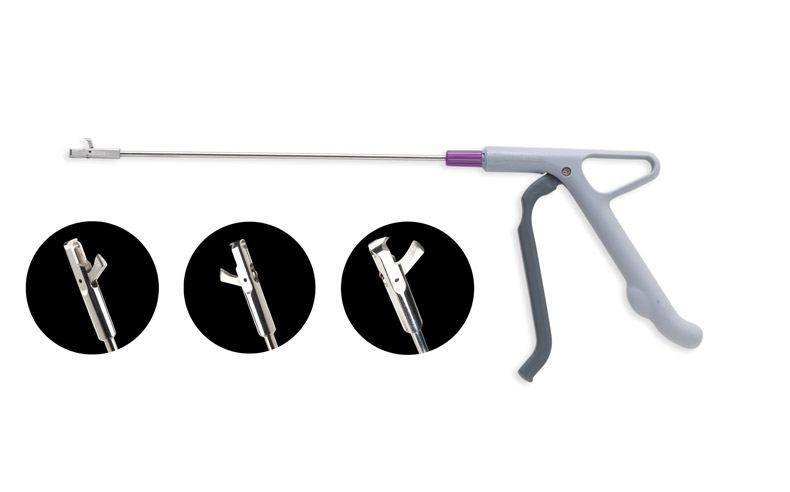
DTR Medical (Llansamlet, UK), a manufacturer of single-use surgical instruments, has specified Sorona, a renewably sourced polymer from DuPont (Le Grand-Saconnex, Switzerland), for the fabrication of six components in its Cervical Rotating Biopsy Punch. DuPont is exhibiting the device at Fakuma this week in Friedrichshafen, Germany.
The 15% glass-filled grade of Sorona EP features high strength, stiffness, and dimensional stability. "The fact that as much as 37% of the material is made with a renewably sourced propanediol (PDO) made from technical starch and that it withstands gamma sterilization are further attributes that appealed to the customer," Rémi Daneyrole, Marketing Communications, EMEA, told PlasticsToday at the DuPont stand.
The Cervical Rotating Biopsy Punch is used to take tissue samples from patients for analysis.
 Sorona EP, which fully complies with the regulatory requirements of healthcare applications and is produced according to Good Manufacturing Practices, is used in the handle and trigger mechanism. The left, right, and front handle components, connector pin, rotational controller, and rotational controller with chamfer, all molded from Sorona EP, are used to activate a spring and drive the inner rod to generate a clamping force to cut the tissue sample.
Sorona EP, which fully complies with the regulatory requirements of healthcare applications and is produced according to Good Manufacturing Practices, is used in the handle and trigger mechanism. The left, right, and front handle components, connector pin, rotational controller, and rotational controller with chamfer, all molded from Sorona EP, are used to activate a spring and drive the inner rod to generate a clamping force to cut the tissue sample.
The single-use device eliminates the potential for cross contamination that may occur when hard-to-clean instruments are used on patients undergoing cervical cancer biopsies. The design saves considerable time and cost compared with reusable devices that must be sterilized.
"The surface finish that can be achieved with Sorona also appealed to the customer," says Daneyrole. Indeed, Andrew Davidson, Managing Director at DTR Medical, noted that the surface finish of the handle is "fundamental as a stainless-steel replacement for instrument quality and [to ensure a] good grip in the clinical setting." The resin has a five-year shelf life, a medical industry requirement, adds Daneyrole.
Another advantage of the material is its similarity to PBT and PET—Sonora is a poly (trimethylene teraphthalate), or PTT, polymer. "Because the properties of the material are similar to PET, no new tooling investment is required for the manufacturer," says Daneyrole. Additional material properties include minimal shrinkage and warpage, and scratch resistance in finished parts, according to DuPont.
The development time for this project was substantial, as is typical in medical applications, notes Daneyrole, and the need for a long-term view is not always understood by staff unfamiliar with medtech development practices. But, the patience has been rewarded.
"This application shows once again that we are all about helping to bring superior products to market and showing our commitment," says Daneyrole. The biopsy punch may be the first medical application for Sorona, but Daneyrole is betting that it won't be the last.
Norbert Sparrow is Senior Editor at PlasticsToday. Follow him on twitter @norbertcsparrow and Google+.
About the Author(s)
You May Also Like




