An at-home cancer screening test developed by Exact Sciences (Madison, WI) has been named a finalist in the prestigious Medical Design Excellence Awards. Cologuard is the first and only FDA-approved noninvasive screening test for colorectal cancer, and could have a significant impact on early detection of colon cancer. Global injection molder and contract manufacturer Phillips-Medisize (Hudson, WI) collaborated with Exact Sciences on the design for manufacture of the collection kit, and evaluation of the user interface and closure system.
May 26, 2015
An at-home cancer screening test developed by Exact Sciences (Madison, WI) has been named a finalist in the prestigious Medical Design Excellence Awards. Cologuard is the first and only FDA-approved noninvasive screening test for colorectal cancer, and could have a significant impact on early detection of colon cancer. Global injection molder and contract manufacturer Phillips-Medisize (Hudson, WI) collaborated with Exact Sciences on the design for manufacture of the collection kit, and evaluation of the user interface and closure system. It also manufactures and assembles the patient-ready kits.
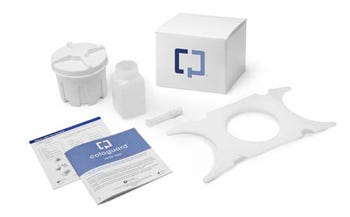 Colon cancer is one of the deadliest forms of cancer if it is discovered in late stages, which happens far more often than it should. Only about 50% of people are willing to undergo a regular colonoscopy, according to Exact Sciences CEO Kevin Conroy. Cologuard removes many of the barriers that keep people away from regular screenings.
Colon cancer is one of the deadliest forms of cancer if it is discovered in late stages, which happens far more often than it should. Only about 50% of people are willing to undergo a regular colonoscopy, according to Exact Sciences CEO Kevin Conroy. Cologuard removes many of the barriers that keep people away from regular screenings.
Using the Cologuard kit, the patient collects a stool sample at home without any dietary restrictions or bowel preparation and sends it back to the Exact Sciences lab in a prepaid mailer. Results are made available in as little as two weeks. Given the contents of the kit and the mode of shipment, zero-defect sealing was a high priority.
"A lot of work was done on the sealing system for the specimen container," Bill Welch, Chief Technology Officer at Phillips-Medisize, told PlasticsToday. "The seal had to stand up under both vacuum and pressure. The specimen is shipped via air travel, so no leakage is permissible under any orientation." An extensive process that involved finite element analysis, rigorous material selection and analysis and test engineering was needed to get this right, adds Welch. "We needed to find a suitable elastomer, which entailed testing a range of durometers and configurations," says Welch, adding that the seal had "to screw on perfectly. It couldn't wander or pinch."
The winners of the Medical Design Excellence Awards will be announced at MD&M East, co-located with PLASTEC East, in New York City on June 9, 2015. The exhibition and conference runs from June 9 to 11. |
Fluid dispensing also was a challenging aspect, according to Welch. "Different chemistries are involved, and the fluid dispensing must be extremely precise," he says. Phillips-Medisize's expertise in drug-delivery systems, which represent a significant part of its business, stood it in good stead. "The technology is complementary to diagnostics in terms of precision molding and dispensing requirements, with similar quality systems and controls," he explains.
Phillips-Medisize has installed a lean manufacturing cell where it provides assembly, kitting, labeling and packaging services for Exact Sciences. The contract manufacturer's breadth of capabilities and its global footprint brought it this business. "We have a full range of product development, engineering and manufacturing services to help commercialize the product. Also, the product may have regional models—for Asia/Pacific, North America and so forth—and our global capabilities are a real advantage in fulfilling customer needs," says Welch.
Beyond the business aspect, Phillips-Medisize is delighted to be a part of this project because of what it represents in the healthcare paradigm, says Welch. "Cologuard represents exactly what is driving healthcare today: Less time in the doctor's office, less cost [Cologuard costs approximately $600, compared with a colonoscopy, which runs about $2000] and less stress for the patient."
The medical technology industry's premier awards program, the Medical Design Excellence Awards honor the achievements of medical device manufacturers, their suppliers and the many people behind the scenes who are responsible for advancing healthcare innovation. The winners of the 2015 MDEA program will be announced at the MD&M East conference and exhibition in New York City on June 9, 2015.
To view a slide show featuring all of the finalists of the 2015 MDEA program, go to the MD+DI website.
Norbert Sparrow is Editor in Chief at PlasticsToday. Follow him on twitter @norbertcsparrow and Google+.
About the Author(s)
You May Also Like




