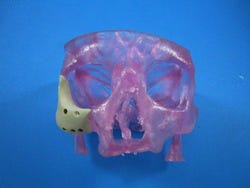
Earlier this month, Oxford Performance Materials Inc. (OPM; South Windsor, CT) received 510(k) clearance from FDA for its 3D-printed OsteoFab Patient-Specific Facial Device (OPSFD). It is the first and, thus far, only 3D-printed polymeric implant for facial indications cleared by FDA, says OPM. The announcement follows FDA clearance in February 2013 of OPM's OsteoFab Patient-Specific Cranial Device.
August 27, 2014
Earlier this month, Oxford Performance Materials Inc. (OPM; South Windsor, CT) received 510(k) clearance from FDA for its 3D-printed OsteoFab Patient-Specific Facial Device (OPSFD). It is the first and, thus far, only 3D-printed polymeric implant for facial indications cleared by FDA, says OPM. The announcement follows FDA clearance in February 2013 of OPM's OsteoFab Patient-Specific Cranial Device.
 "There has been a substantial unmet need in personalized medicine for truly individualized—yet economical—solutions for facial reconstruction, and FDA's clearance of OPM's latest orthopedic implant marks a new era in the standard of care for facial reconstruction," said Scott DeFelice, OPM Chief Executive Officer and Chairman, in a prepared statement. "Until now, a technology did not exist that could treat the highly complex anatomy of these demanding cases. With the clearance of our 3D-printed facial device, we now have the ability to treat these extremely complex cases in a highly effective and economical way, printing patient-specific maxillofacial implants from individualized MRI or CT digital image files from the surgeon. This is a classic example of a paradigm shift in which technology advances to meet both the patient's needs and the cost realities of the overall healthcare system."
"There has been a substantial unmet need in personalized medicine for truly individualized—yet economical—solutions for facial reconstruction, and FDA's clearance of OPM's latest orthopedic implant marks a new era in the standard of care for facial reconstruction," said Scott DeFelice, OPM Chief Executive Officer and Chairman, in a prepared statement. "Until now, a technology did not exist that could treat the highly complex anatomy of these demanding cases. With the clearance of our 3D-printed facial device, we now have the ability to treat these extremely complex cases in a highly effective and economical way, printing patient-specific maxillofacial implants from individualized MRI or CT digital image files from the surgeon. This is a classic example of a paradigm shift in which technology advances to meet both the patient's needs and the cost realities of the overall healthcare system."
The OPSFD will be 3D printed by medical device OEM OPM Biomedical using the OsteoFab process, which combines laser sintering additive manufacturing technology and OPM's proprietary OXPEKK powder formulation to print orthopedic and neurological implants. These implants are biocompatible, mechanically similar to bone, radiolucent, and support bone attachment, according to the company.
Biomet Inc., a distributor of advanced technologies for the treatment of arthritis, joint and spine related injuries, and facial reconstruction headquartered in Warsaw, IN, will be the exclusive global distributor of OPM's OPSFD.
Is this the dawn of a new age, when every hospital bed will come with a 3D printer, as some industry observers have speculated? Not so fast, says Linda Tian, medical device analyst at research and consulting firm GlobalData. The lack of insurance coverage for patient-specific implants and insufficient reimbursement for complex trauma cases are deterring many craniomaxillofacial (CMF) surgeons from participating in medical training for using presurgical planning and 3D-printed implants, she said.
The technology is not the issue. "The processing chain, from data acquisition to 3D printing of CMF patient-specific implants, has proven to be practical and uncomplicated," said Tian in a press release. "However, 3D printing might continue to be plagued by a major weakness in terms of its future growth within the orthopedic industry, namely the need for hospital administrators to cut costs associated with high-volume surgeries, such as trauma."
While 3D-printed implants may theoretically reduce the overall cost of the procedure by reducing surgery and inpatient time and minimizing complications, clinical evidence of the actual cost effectiveness of 3D-printed implants in CMF surgeries is lacking, noted Tian.
Nevertheless, GlobalData expects that OPSFD's approval will lead to a further rise in the use rate of related custom devices. "Over the next five years, the medical devices sector will see more partnerships between small contract 3D-printing service firms and large orthopedic companies seeking to explore opportunities in this revolutionary technology," said Tian.
About the Author(s)
You May Also Like




