Bacteria love company. Once they gain a foothold in the body, they like to form colonies, called biofilms. Invasive and implantable medical devices are prime real estate for these colonies that cause infections and life-threatening conditions. Now, researchers at the Wyss Institute for Biologically Inspired Engineering (Boston) have developed polymers that store considerable amounts of lubricating liquids within their molecular structure and release them over time to render the material continuously slippery and repellant to bacteria.
February 17, 2015
Bacteria love company. Once they gain a foothold in the body, they like to form colonies, called biofilms. Invasive and implantable medical devices are prime real estate for these colonies that cause infections and life-threatening conditions. Now, researchers at the Wyss Institute for Biologically Inspired Engineering (Boston) have developed polymers that store considerable amounts of lubricating liquids within their molecular structure and release them over time to render the material continuously slippery and repellant to bacteria. An experiment using a solid silicone polymer that is typically used in medical tubing infused with a silicone oil is described in the current issue of ACS Biomaterials Science & Engineering.
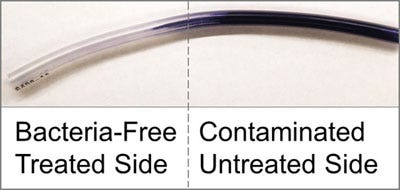 "The solid silicone tubing is saturated with silicone oil, soaking it up into all of the tiny spaces in its molecular structure, so that the two materials really become integrated into one," says Caitlin Howell, a co-author of the article. In addition to imparting a non-welcoming surface to bacteria, the saturation process produces a liquid-infused polymer that is sufficiently robust to withstand conventional sterilization methods.
"The solid silicone tubing is saturated with silicone oil, soaking it up into all of the tiny spaces in its molecular structure, so that the two materials really become integrated into one," says Caitlin Howell, a co-author of the article. In addition to imparting a non-welcoming surface to bacteria, the saturation process produces a liquid-infused polymer that is sufficiently robust to withstand conventional sterilization methods.
In the test described in the article, the researchers exposed treated and untreated medical tubing to Pseudomonas aeruginosa, E. coli and Staphylococcus epidermis, which frequently form biofilms and cause urinary, tissue and blood infections. The experiment confirmed that the liquid-infused polymer tubing greatly reduced bacterial adhesion and largely eliminated biofilm formation, according to the researchers.
|
Graphic of CRE bacteria courtesy Centers for |
Preventing biofilm formation on medical devices has become critically important, as the bacteria are extremely difficult to dislodge once they have settled in and are increasingly resistant to antibiotics.
"With widespread antibiotic resistance cropping up in many strains of infection-causing bacteria, developing out-of-the-box strategies to protect patients from bacterial biofilms has become a critical focus area for clinical researchers," says Wyss Institute Founding Director Donald Ingber, M.D., PhD, in a news release from Wyss. "Liquid-infused polymers could be used to prevent biofilms from ever taking hold, potentially reducing rates of infection and, therefore, reducing dependence on antibiotic use."
About the Author(s)
You May Also Like