There’s no way around it: You have to use the right equipment if you want an accurate moisture measurement.
October 26, 2010
There’s no way around it: You have to use the right equipment if you want an accurate moisture measurement.
Let’s start by defining the requirements for a moisture analyzer that is suitable for a production environment. First, it has to be accurate and precise. For those who did not have the benefit of training in statistical process control or who may have forgotten the key points, accuracy refers to the agreement that the instrument gives with the actual value.
|
This is where calibration comes in. Standards that provide a known outcome are used to determine if instruments are providing the right answer. Precision is indicated by results that are repeatable. Often the two are confused, and repeatability is cited as evidence for a correct answer. However, a series of measurements may be close to each other but not in good agreement with the correct answer.
Precision involves control over the experimental parameters—temperature, time, sample size, etc. Accuracy, in the area of moisture measurement, involves being sure that all of the moisture in the raw material is extracted and detected and that no other products that evolve from the plastic are confused with the water as the polymer is being heated. After all, this is moisture analysis, not volatiles analysis. Any device used to measure moisture content must be able to ensure complete removal of moisture from the sample quickly. If the instruments are to be worthwhile in a production environment, they should be able to perform the task of complete moisture removal in 10-20 minutes.
How do we know when we have extracted all of the water from a sample? There actually is an ASTM method that addresses this, although for some arcane reason the method has a title that suggests that it is written specifically for nylon. However, the principle works for all plastic materials. The method involves heating a sample at a particular temperature and obtaining a result.
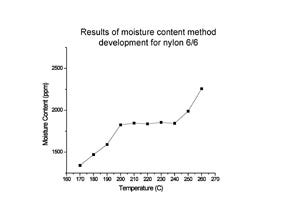
The test temperature is then increased by some increment, usually 10 deg C, and the test is repeated on a new sample of the material. If the value obtained keeps increasing with temperature, then the temperature is not yet high enough to remove all of the moisture.
At some point, the value will level off with increasing temperature. This indicates that all the water has been removed from the sample. We should expect that the correct test temperature will be quite high, well above the temperature used to dry the material in preparation for molding. For example, it can take a resin dryer 3-4 hours to dry polycarbonate (PC) down to a moisture content of 0.020% at a drying temperature of 120°C. So it should not be surprising to find out that removing all of the moisture from a PC sample in just a few minutes requires a temperature of approximately 200°C, a temperature at which the material will soften.
|
The range for the plateau that forms when moisture content is plotted with temperature may be limited. Some materials, such as nylon, will undergo solid-state polymerization if heated to a particular temperature. This process actually creates water, which will be indistinguishable from water that was actually in the sample. Therefore, staying within a particular temperature region for each polymer type is important. Figure 1 shows this principle for an actual method development exercise using a Karl Fischer titrator.
A simple check can be run to determine if a test has successfully removed all the moisture from a sample. After running the initial test and obtaining a value, simply insert the sample back into the heating chamber at a temperature 10 deg C higher than the first test without allowing it to come in contact with the atmosphere between the time the first test ends and the second one begins. The second test should produce a value of zero.
Too many variables
If you have a loss-on-drying (LOD) analyzer, try to perform the exercises above. First, take a particular material and run tests at progressively higher test temperatures. Look for the point at which the plateau occurs. Start early in the morning and clear your schedule, because it is going to be a long day. The plateau will never occur. This is because LOD systems do not simply measure water content; they quantify all volatiles that can come off at any given temperature.
All those other peaks in the pyrolysis-mass spectroscopy scan shown in Part 1 of this topic have a particular temperature at which they evolve. Many of them overlap with the water in the sample. As the temperature is increased, the amount of volatile material will steadily increase. Once a particular temperature is achieved, the polymer itself will begin to degrade and the degradation byproducts will add further to the weight loss.
The same lack of resolution will occur if you try to run the same sample twice with a slight increase in temperature on the second run. In a true moisture analyzer, once all the water is removed, there can be no more water to measure on the second test. But if you try the same technique with an LOD system, each incremental increase in temperature will bring an additional weight loss. These two results should be sufficient proof that LOD systems do not work for plastic materials.
It has nothing to do with the apparent lack of sophistication in the LOD instrument. When you look at a Karl Fischer device with its glassware, tubes, and reagents, it looks like a higher technology instrument than the simple heating chamber that constitutes the heart of an LOD instrument. But this is not what makes Karl Fischer workable and LOD systems unsuitable for moisture measurement in plastics. It is the fact that the Karl Fischer chemistry reacts specifically to water and records only that portion of the volatiles that is water.
A lot of other compounds are also being evolved during a Karl Fischer test, but the instrument does not “see” them. An LOD system sees everything that evolves from the material and counts it all as moisture because it has no tools for distinguishing the water from everything else.
TGA exposes LOD shortcomings
Thermogravimetric analysis (TGA) is a very sophisticated method for analyzing the composition of polymers. The instrument cost is five to 10 times that of either an LOD or a Karl Fischer instrument. TGA is used, among other things, to measure weight loss as a function of increasing temperature. The initial weight-loss region is associated with the volatiles in the polymer.
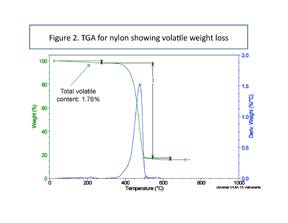
Figure 2 shows a typical result for a 15% glass-filled nylon 6. Note that before polymer decomposition begins at approximately 350°C, nearly 2% of the sample weight has been lost. Some analysts interpret this weight loss as representative of the moisture content of the sample. However, despite the cost and precision of the TGA instrument, it cannot provide an accurate measurement of moisture because it cannot distinguish between the various components that are evolving from the sample as it is heated. The actual moisture content of this sample was found to be less than 0.9%. The remaining weight loss represents the other volatile compounds in the material.
The TGA in Figure 2 may not be a fair comparison with a moisture test because the TGA sample is exposed to a continually increasing temperature, while moisture content tests are performed at a constant temperature.
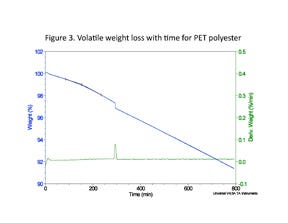
To provide a true apples-to-apples comparison, we need to look at a TGA result from an isothermal test. This is shown in Figure 3 for a PET polyester. In this case, the weight loss is plotted as a function of time instead of temperature. Note that the weight of the sample never becomes stable. The weight loss process continues indefinitely and the weight loss reported is dependent on the time period over which the test is conducted. Reducing the test temperature will reduce the weight lost, but it is not possible to reach a stable sample mass because there are so many compounds in the polymer that evolve across a wide range of temperatures and are of different volatility.
Given this situation, how does a manufacturer of an LOD system support the claim that the system is calibrated? And what is it calibrated to? The answer to the first question is complicated and a complete explanation will be provided in Part 3, when we will take up the broader issue of instrument calibration and what can go wrong with any measurement technique.
The answer to the second question may be surprising. LOD systems use Karl Fischer titrators as their benchmark. Every manufacturer of LOD analyzers relies on Karl Fischer titrators to provide them with a reality check on what their instruments are doing. But the fact that the two types of instruments can be made to give the same answer does not mean that they are measuring the same thing. This will also be covered in detail in Part 3.
The facts, please
To close, let’s return to the list of statements made about moisture analyzers cited in Part 1 and give the complete picture.
• Karl Fischer titration uses expensive chemicals that are toxic. The device does use chemicals and for a system that operates in a production environment where use can be heavy, the cost of reagents will range from $1500-$2500/year. The concerns about toxicity come from the fact that one of the chemicals in the Karl Fischer cocktail used to be pyridine, which is toxic. However, as far back as the mid-1980s, pyridine-free reagents were being sold and today they are the norm. Nevertheless, these are chemicals and need to be handled with appropriate care. One of the major ingredients is methanol, so drinking is not advised. But toxicity has been addressed.
• Karl Fischer equipment includes glassware that can break. True. You cannot operate around a piece of lab equipment with the same mindset that you operate around a large piece of machinery.
• Karl Fischer titration is too difficult for the average manufacturing person to master. False. When our lab purchased our first Karl Fischer system in 1986, it did take some time to learn the instrument, largely because there was very little information available on correct settings for plastic materials. We found ourselves caught between two worlds: the makers of the instrument who knew a lot about the science of moisture measurement but almost nothing about polymers, and the manufacturers of the plastic materials who were expert in the requirements for moisture removal before processing but had little or no experience with actual moisture measurement.
However, once we systematically figured things out, personnel on all three shifts were trained to run the instrument. The lab people were still responsible for maintenance and calibration, just as in a typical plant you have maintenance people who specialize in the upkeep and repair of the molding machines and auxiliary equipment. But many members of the company could use the equipment. And when a second plant was opened in Mississippi, the same type of instrument was installed there and was used successfully around the clock. Any technician capable of understanding the complexities of the injection molding process has already demonstrated the mental capacity needed to operate a Karl Fischer titrator.
• Loss-on-drying systems are calibrated to produce moisture measurements that are as precise as those obtained using the more sophisticated lab equipment. Precise, perhaps. Accurate, not necessarily. And as we will see next month, even if the numbers are the same, the things being measured are not.
• Ongoing calibration of loss-on-drying systems is not necessary. In a world where we calibrate our gauge pins and verniers, pieces of equipment that are static most of the time, is there anyone who really believes the proposition that an instrument that contains a heating element and a sensitive analytical balance requires no calibration?
More to come.
About the Author(s)
You May Also Like