October 6, 2016
When Dr. Frank Ruttens, of Agfa-Labs (Belgium) talks about Hansen Solubility Parameters, his face lights up as he starts drawing circles and diagrams. Agfa-Labs is the open innovation platform of Agfa Gevaert NV for materials and coatings research.
“We are a research department with about 150 people, and we offer R&D services to third parties. We make use of advanced research tools such as High Throughput Formulation and High Throughput Screening, but our unique selling point is our expertise on Hansen Solubility Parameters (HSPs). We can determine the HSP values of unknown substances.”
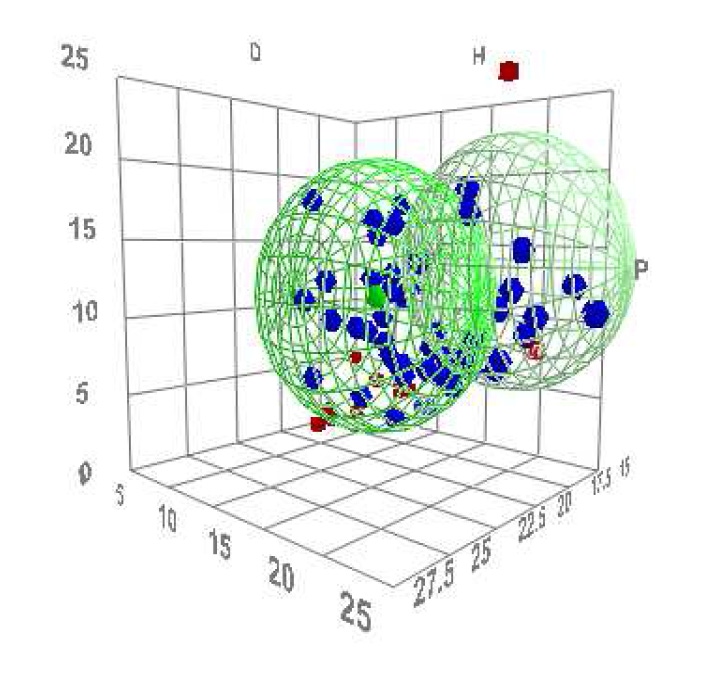
 Why is that interesting? And what are Hansen Solubility Parameters, anyway? Hansen solubility parameters were developed by Charles M. Hansen in his Ph.D thesis in 1967 as a way of predicting solubility behavior. Will a solvent ‘work’ - will it dissolve and form a solution with a particular material or not? Hansen developed a model based on three component parameters - dispersion, polar and hydrogen bonding – the coordinates of which are represented as a dot in a 3-dimensional space, each dot being surrounded by what is known as a solubility sphere. The radius of the sphere is called the interaction radius. These spheres are the circles drawn by Dr. Ruttens, to illustrate what he means.
Why is that interesting? And what are Hansen Solubility Parameters, anyway? Hansen solubility parameters were developed by Charles M. Hansen in his Ph.D thesis in 1967 as a way of predicting solubility behavior. Will a solvent ‘work’ - will it dissolve and form a solution with a particular material or not? Hansen developed a model based on three component parameters - dispersion, polar and hydrogen bonding – the coordinates of which are represented as a dot in a 3-dimensional space, each dot being surrounded by what is known as a solubility sphere. The radius of the sphere is called the interaction radius. These spheres are the circles drawn by Dr. Ruttens, to illustrate what he means.
“The principle is one that has long been known: like seeks like, and like dissolves like,” he explained. “The HSP of a solvent and that of the solute will be close together. We can determine how close together the spheres are, or how far apart.”
He added: “Performance is affected by HSP. The shorter the HSP distance, the more likely they are to be compatible and therefore the better the solubility. Simply put: in a blend, two polymers must like each other, or they won’t bond. Another example would be a plasticizer, which has to stay in the polymer matrix. It’s about controlling solubility.”
“But if we can identify the solvents on the basis of their HSP, we can create designer solvents by mixing solvents together that are tailored to a specific target material.”
The HSP used to be determined by exposing a test material to a vast range of different solvents to find out which were good and which were bad. Today, much of these data can be predicted with the help of software. Agfa-Labs originally used the technology for the development of UV curable inks, and is now offering it to their clients. It is a tool that can be used in a huge variety of applications, in sectors ranging from the petrochemical and pharmaceutical industry to green solvents, coatings, polymer processing and nanotechnology. In one case, it enabled them to replace a toxic solvent by a more environmentally-friendly solvent - with the exact same HSP. “We took one out, and put another one in,” said Ruttens. “It was actually a question of simple plug and play.”
There are still large numbers of substances for which the parameters are unknown. The HSP of many bioplastics, for example, remain to be determined. Ruttens: “Yet once we determine the HSP values, we can make accurate predictions about which building blocks will work together. It means that much less lab time is needed, reducing costs, improving performance – and considerably speeding up the time to market for new formulations.”
About the Author(s)
You May Also Like


