3D printing processes and 3D modeling software became critical tools in a groundbreaking face and hands transplant operation.
February 7, 2021
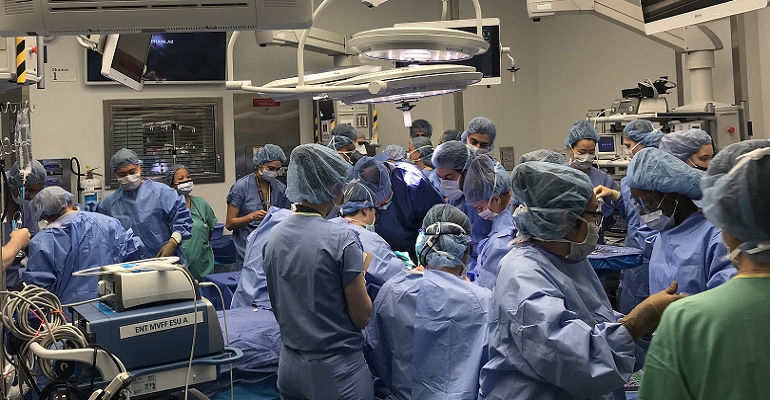
Almost six months after a rare face and hands transplant, 22-year-old New Jersey resident, Joe DiMeo, stepped out to show how the transplant now allows him to smile, blink, pinch, and squeeze. DiMeo had the operation last August, two years after being badly burned in a car crash that left him with severe injuries to his face and both arms. He suffered significant damage to his soft tissue, which severely limited his ability to lead a normal life.
During a preparation period of 14 months, Materialise clinical engineers formed a cohesive team alongside NYU Langone surgeons, rehearsing the transplant operation in a lab environment to develop and fine-tune the surgical plan. Once a suitable donor was found, the team, led by Dr. Eduardo Rodriguez, professor of reconstructive plastic surgery the Helen Kimmel, chair of the Hansjörg Wyss Department of Plastic Surgery at NYU Langone, had only 24 hours to begin the procedure that would improve the DiMeo’s function, appearance, and quality of life.
Materialise offered the team 3D planning and printing tools to enable the speed and accuracy required for this complex medical procedure. 3D printed personalized tools such as those used in the double hand and face transplant, are also increasingly common for use in routine surgery, providing surgeons with an additional level of confidence which results in improved patient outcomes.
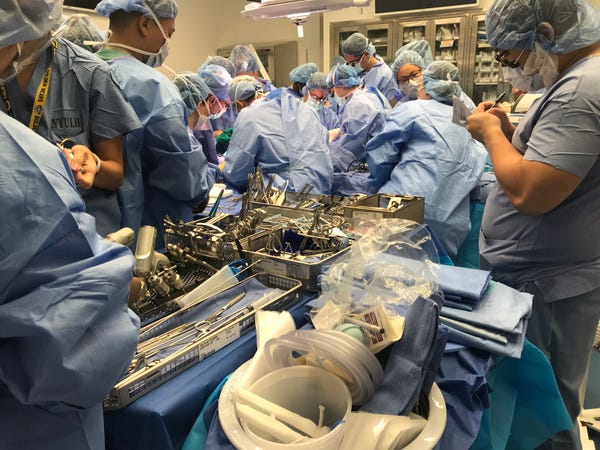
Creating the Tools for the Operation
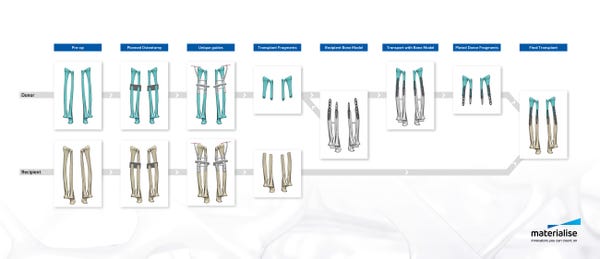
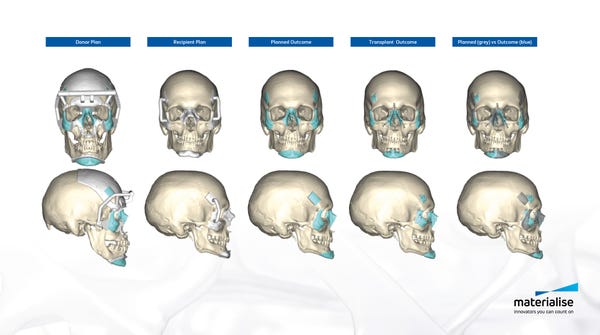
During the procedure, Rodriguez and his surgical team of sixteen used Materialise’s 3D printed cutting and drilling guides. “We used several medical tools for this procedure. The cutting guides used for this operation allowed surgeons to cut the donor and recipient bones very precisely. We also used drilling guides that allow the donor bones to be attached to the recipient's bones more easily and with great accuracy,” Kristof Sehmke, global communication manager at Materialise Medical, told Design News. “These cutting and drilling guides are prepared virtually based on CT scans and the surgeon’s indications, as part of the pre-surgical planning.”
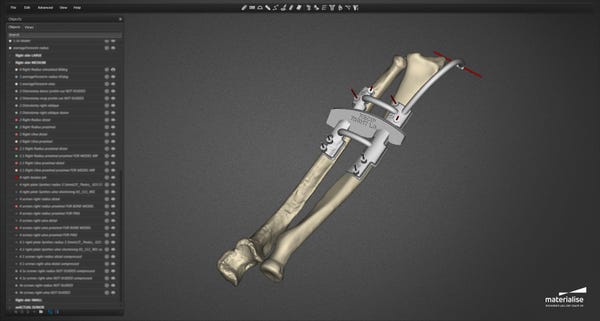
In creating the tools for the operation, Materialise worked with the medical team to match the tools with the patient’s physiology. “The patient-specific guides follow the personal anatomy of the donor and recipient. The guides are then 3D printed and shipped to the hospital,” said Sehmke. “While these guides are entirely patient-specific, this type of personalized surgical tools is not new and also increasingly used for more routine surgery, providing increased speed and accuracy.”
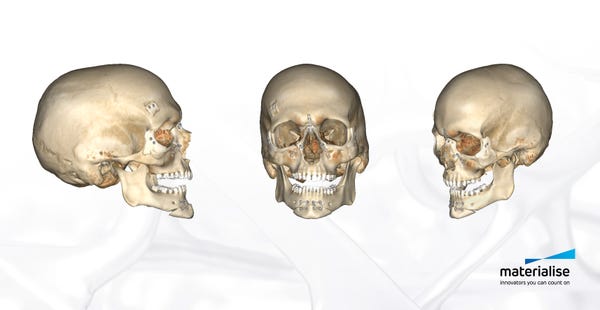
In the months leading up to the surgery Materialise engineers coordinated the development of a surgical plan and created an on-screen 3D model based on CT-scans. This allowed the surgeons and clinical engineers to virtually plan the procedure and visualize different scenarios in three dimensions, creating an in-depth understanding of the anatomical bone structure and determining the optimal surgical flow. “The pre-surgical planning consisted of a virtual 3D on-screen representation and simulation of the planned surgery, providing surgeons with detailed insights and an additional level of confidence before entering the operation room,” said Sehmke. “The surgeons created 3D printed surgical cutting and drilling guides. The guides provide increased speed and accuracy. They are typically used for hip, knee, and shoulder surgery but also for cranio maxillofacial surgery.”
Bringing FDA Approval to the Process
As for the choice of Materialise as a software source for the operation, there were several tools the company was able to provide to the surgical team. “The medical industry is highly regulated and has very strict safety requirements. Materialise has pioneered many leading medical applications of 3D printing and personalized solutions have been Materialise Medical’s core business since its foundation in 1990,” said Sehmke. “We bring nearly three decades of experience in developing medical solutions that help researchers, engineers and clinicians achieve the desired patient outcomes,”
As well as providing cutting-edge technology to the surgical team, Materialise also brought the credibility of an FDA blessing. “The company has a legacy of adhering to the highest possible safety standards and has obtained CE Marking Certification for most of its personalized orthopedic and cranio maxillofacial solutions. This includes 3D-printed anatomical models and patient-matched surgical guides and implants,” said Sehmke. “Materialise was also the first company in the world to receive FDA clearance for software intended for 3D printing anatomical models for diagnostic use. This kind of certification helps raise the industry bar to ensure patient safety and transparency on personalized devices' production.”
Rob Spiegel has covered manufacturing for 19 years, 17 of them for Design News. Other topics he has covered include automation, supply chain technology, alternative energy, and cybersecurity. For 10 years, he was the owner and publisher of the food magazine Chile Pepper.
About the Author(s)
You May Also Like




