July 5, 2016
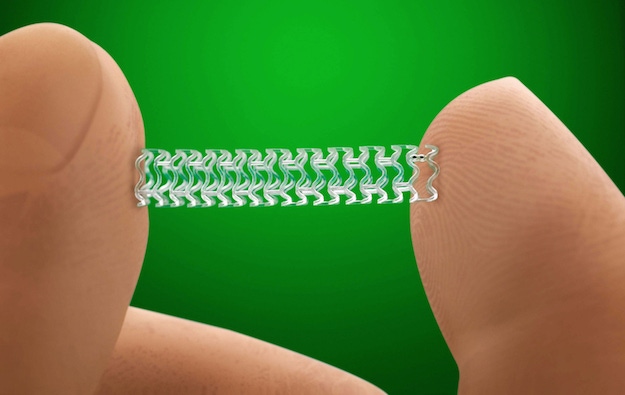
FDA today approved the first fully absorbable stent to treat coronary artery disease. The Absorb GT1 Bioresorbable Vascular Scaffold System (BVS) from Abbott Vascular (Santa Clara, CA), which releases the drug everolimus to limit the growth of scar tissue, is manufactured from poly(L-lactide). The bioresorbable polymer is gradually absorbed by the body in approximately three years.
“FDA’s approval of the Absorb GT1 BVS offers a new treatment option for individuals who are candidates for angioplasty, but would prefer an absorbable device rather than a permanent metallic coronary stent,” said Bram Zuckerman, MD, Director of the Division of Cardiovascular Devices at FDA’s Center for Devices and Radiological Health.
Poly(L-lactide) is similar to materials used in other types of absorbable medical devices, such as sutures. The device’s absorption by the body gradually eliminates the presence of foreign material in the artery once the stent is no longer needed. After absorption, there are only four very small platinum markers embedded in the walls of the artery, which help cardiologists identify where the stent was originally placed.

The company initially hoped to receive FDA approval for the device as early as 2012. (The device received the CE mark in January 2011 and is currently available in more than 100 countries.) “The long and winding road since [2011] says a lot about how innovations happen, and about just how hard it can be,” writes Amy Feldman in Forbes. Abbott, like other manufacturers of bioabsorbable polymer stents, was challenged with the “twin problems of strength and absorption.” Polymers aren’t as strong as metals, and when you try to make them strong they become brittle, notes Feldman. The company also had to engineer an optimal resorption rate. “If the stent dissolved too quickly, the artery might not remain open long enough, but if it went away too slowly it wouldn’t offer as much advantage over a traditional stent,” writes Feldman.
In approving the Absorb GT1 BVS, FDA evaluated data from a randomized trial of 2,008 patients, which compared the rate of major adverse cardiac events between the Absorb GT1 BVS and a drug-eluting metallic stent, notes the agency in a press release announcing approval of the device. After one year, the Absorb GT1 BVS group showed a major cardiac adverse event rate of 7.8%, which was clinically comparable to the rate of 6.1% observed in the control group. In addition, after one year, the rate of blood clots forming within the devices was 1.54% for the Absorb GT1 BVS and 0.74% rate for the control.
Coronary heart disease is responsible for about 370,000 deaths each year in the United States, according to the National Heart, Lung, and Blood Institute. Angioplasty is typically performed to widen the artery using a metal stent. However, scar tissue can form within the stent causing the artery to narrow again. Drug-eluting stents temporarily release a drug, typically for a few months after stent placement, to combat the formation of scar tissue.
By contrast, bioresorbable stents are designed to leave the body over a period of time and allow the artery to heal itself in lieu of relying on a permanent metal structure.
About the Author(s)
You May Also Like




