Sponsored By
Medical
ultrasonic welder
Auxiliaries
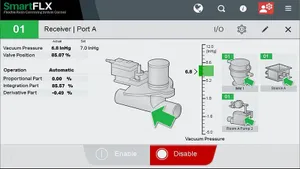
Next-gen Ultrasonic Welders Have Plastics FixationNext-gen Ultrasonic Welders Have Plastics Fixation
Rinco Ultrasonics aims to show NPE attendees how they can improve efficiency and productivity with next-gen pneumatically driven ultrasonic welding technology.
Sign up for the PlasticsToday NewsFeed newsletter.