Congratulations are in order for Phillips-Medisize (Hudson, WI), which won a Gold Award in the Diagnostic Products and Systems category at the 18th Annual Medical Design Excellence Awards (MDEA) ceremony in New York City. The company was cited for its role as an outsourced design and manufacturing partner of Exact Sciences Inc. (Madison, WI) in the development and manufacture of Cologuard, the first and only FDA-approved noninvasive stool DNA screening test for colorectal cancer. Cologuard is prescribed by a physician and sent to the patient’s home.
June 12, 2015
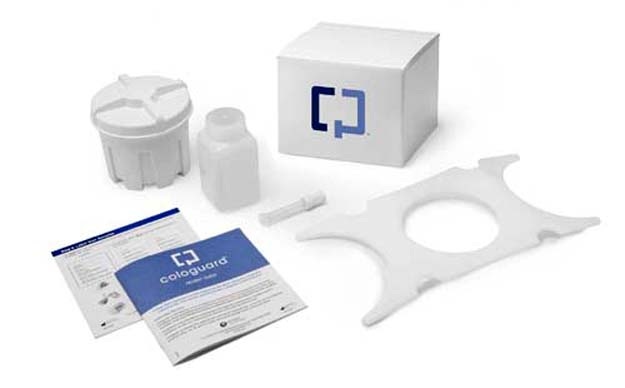
As reported in PlasticsToday earlier this month, Phillips-Medisize worked with Exact Sciences on design for manufacture, along with an evaluation of the user interface and closure of the collection kit. Phillips-Medisize also manufactures and assembles the patient-ready kits. Read "How Phillips-Medisize helped bring award-winning, at-home cancer test to market" for more details on this collaborative, award-winning project.
Produced by UBM Canon, the MDEA program is the medtech industry’s premier design competition, celebrating the achievements of medical device manufacturers, their suppliers and the many people behind the scenes—engineers, scientists, designers, and clinicians—who are responsible for bringing life-saving and quality-of-life-enhancing products to market.
To return to the beginning of this slide show, click here.

About the Author(s)
You May Also Like




