Genesis Medical Plastics and Renolit Healthcare Solutions partner to support development of polyolefin-based medical products.
February 9, 2023
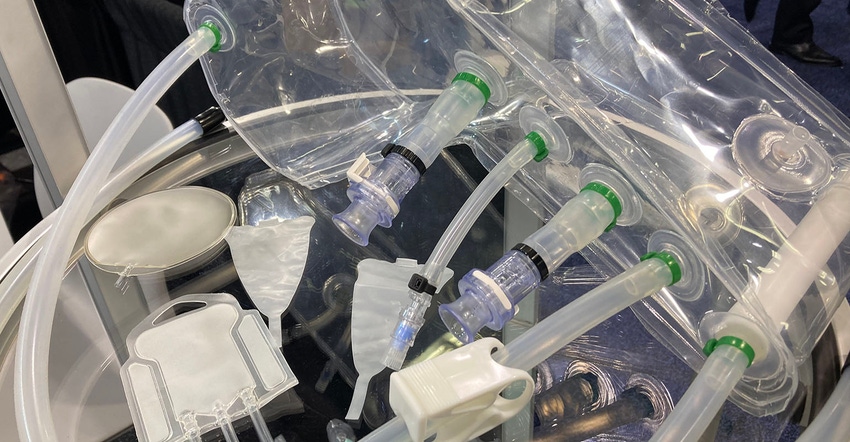
The use of PVC in medical applications has a long history, and a very successful one at that. The phase-out of potentially harmful chemicals in the European Union by 2030, however, may affect future use of PVC in healthcare products. It’s been in the conversation in the United States for 30-plus years, acknowledged Tom Ryder, President and CEO of Genesis Plastics Welding. “It comes and goes,” he added, noting that shifting to a non-PVC material in an existing product is costly when it comes to retooling production lines and arduous in terms of validation. But companies supplying the vast EU market will need to consider making a change, and that might trickle down to US medical device manufacturers. Through its relationship with materials supplier Renolit Healthcare Solutions, Genesis Plastics Welding intends to be ready if and when that happens.
Genesis currently uses Renolit’s PVC for an array of medical products, including in its Class 7 cleanroom environment, but Renolit Healthcare Solutions is also a market leader for medical-grade non-PVC films, said Genesis. It heat seals and welds the films to produce flexible single-use products such as blood bags, biotechnology products, IV bags, dialysis devices, and components such as ports and caps, multi-layer polyolefin secondary packaging, and other tailor-made solutions.
In particular, the companies collaborate to support OEMs developing new products by introducing them to new materials to future-proof their medical devices and avoid costly re-validation processes. “Polyolefins are a nice option to have,” Ryder told PlasticsToday from the company’s booth at the co-located Plastec West and Medical Design & Manufacturing (MD&M) West event in Anaheim, CA, this week. R&D on the polyolefins is well underway. “We are working with new customers on new products, but it will still take some time for commercialization,” said Ryder. “It could be between eight months and three years, depending on the validation process.” One bioprocessing application is in the pipeline, Ryder added. “We will have a story to share in the near future.”
Needless to say, stay tuned to PlasticsToday for more on this as the story — and application — develop. For now, drop by booth 1946 and check out how Genesis Plastics Welding is supporting medical device OEMs today in their material selection process.
Plastec West and Medical Design & Manufacturing (MD&M) West runs from Feb. 7 to 9, 2023, at the Anaheim Convention Center. The event is organized by Informa Markets – Engineering, which also produces PlasticsToday.
About the Author(s)
You May Also Like




