February 25, 2021
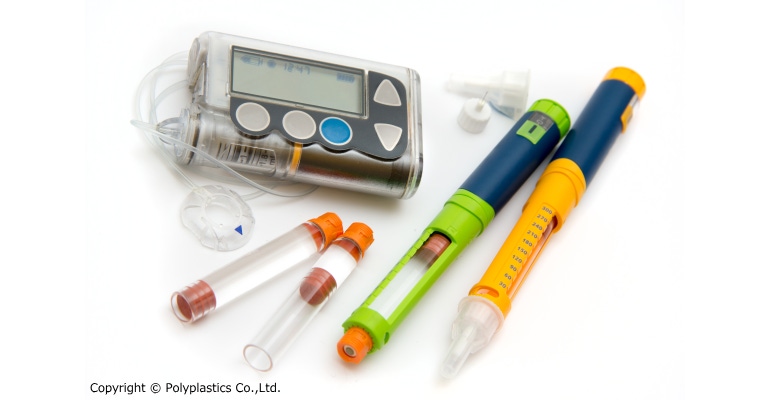
A new high-flow grade of the Duracon polyoxymethylene (POM) PM series has been introduced by the Polyplastics Group. Duracon PM27S01N offers reduced wall thickness, miniaturization, and lower weight for drug-delivery devices that are becoming increasingly complicated and highly functional.
Polyplastics said that its PM series complements the company’s Topas cyclic olefin copolymer (COC) product, a high-purity material for a range of medical applications.
Duracon PM27S01N meets numerous regulatory compliance requirements, including ISO 10993 and USP Class VI biocompatibility/cytotoxicity, FDA Drug Master File (DMF) and Device Master File (MAF), and EU 10/2011 and FDA food-contact 21 CFR 177.2470.
The material adheres to strict quality-management systems including conformity to VDI guideline, VDI 2017 medical-grade plastics, a standard developed by the German Engineer’s Society. Processes and products are fully traceable, and production management is based on GMP principles. Polyplastics also provides uniform quality and global supply.
Polyplastics offers medical device manufacturers extensive data on the long-term reliability of its materials. Customized data on extraction, moldability, durability, slip and wear, and other key attributes are also available.
Topas COC is a glass-clear and highly pure plastic that offers stiffness and barrier resistance, biocompatibility, and drug compatibility for wearables, drug delivery, medical devices, pharmaceutical blisters and trays, and diagnostics and microfluidics.
PlasticsToday recently published an in-depth article on COC's medical applications in its Medical Plastics 101 series, "Cyclic Olefin Copolymer Fulfills Complex Medtech Performance Requirements."
About the Author(s)
You May Also Like


