September 28, 2022
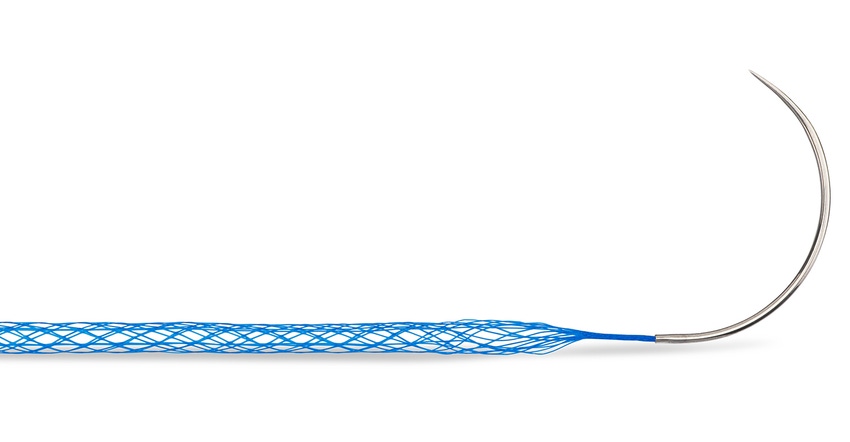
Mesh Suture Inc. (MSi) has received 510(k) clearance from FDA for its Duramesh non-absorbable polypropylene mesh suture. Described by the company as a first-of-its-kind product, Duramesh combines the principle of implant incorporation in a mesh repair with the placement precision of a suture for soft tissue repairs.
The device is designed to mitigate surgical failure caused by suture pull-through. The sharp leading edge of a conventional suture can slice through otherwise intact tissue, potentially leading to dehiscence (the reopening of a surgical incision), hernia formation, and poor tendon function, said MSi. The novel architecture of the Duramesh device flattens at the suture-tissue interface to prevent pull-through. Its open-walled hollow design also allows tissue ingrowth for implant incorporation. In a porcine study, Duramesh reportedly had numerically fewer loose sutures and hernias in comparison to a conventional suture.
“Duramesh offers the perfect combination of strength and simplicity in a surgical repair,” said Dr. Gregory Dumanian, Chief Medical Officer at MSi. “It combines the handling characteristics of traditional suture with a mesh polyfilament design. We are excited to bring this innovative technology to our surgeon colleagues and to their patients who need it most. By designing Duramesh to address the surgical complication of suture pull-through, we expect to see sizeable improvements in patient outcomes,” said Dumanian.
Duramesh received the CE mark in 2021 and is currently in clinical use in the European Union and United Kingdom. It is the first device developed by MSi, which benefits from decades of clinical research and patient care at Northwestern University’s Feinberg School of Medicine in Chicago.
You May Also Like


