The Pixie pen is automatically assembled from 14 components. Of those, Quadrant CMS injection molds six plastic components using five different engineering plastics.
December 13, 2016
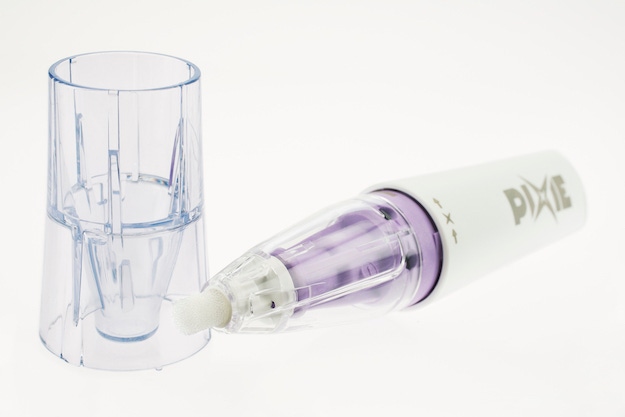
Quadrant Creative Moulding & Systems (Quadrant CMS; Tielt, Belgium) collaborated with pharmaceutical company Oystershell Laboratories (Drongen, Belgium) in the development of a safe, easy-to-use wart removal device. The Pixie pen, which doses small amounts of nitrous oxide onto the skin from a pressurized container, is automatically assembled from 14 components. Of those, Quadrant CMS injection molds six plastic components using five different engineering plastics, including acetal, polycarbonate, nylon, PMMA and SAN.
Key components of the device must withstand high pressures, remain gas tight and accommodate an ultra-low application temperature (-80°C) as the nitrous oxide expands from liquid to gas and freezes the targeted wart. Current products for freezing warts at home involve canisters with a dangerous mixture of liquefied propane gas and dimethylether. The Pixie pen, which can be used for up to eight treatments, has an attractive price point and will revolutionize the current market, says Bart Rossel, CSO at Oystershell.
The Pixie pen began appearing in pharmacies in Belgium a few weeks ago. Oystershell has licensed the rights for the product to a top-five global pharmaceutical company for the whole of Europe; North American and Asian markets will follow soon.

Product development, warts and all
The first phase of the project involved an intensive study by a special Quadrant CMS development team, working in close collaboration with medical researchers at Oystershell and experts from external suppliers. The study involved input on engineering, production, quality control, purchasing and sub-assembly.
Prototype dispensers were assembled from machined plastic parts that were made in collaboration with another Quadrant company, Quadrant Engineering Plastic Products, also in Tielt. Exactly one year after the start of the project, Quadrant produced the first pen using components it had injection molded on prototype tools.
The partners had to take several critical aspects into account during the development process:
Controlling the flow of gas held under high pressure (at least 50 bar) in a cartridge;
providing protection from overheating;
managing a total of 16 equipment and parts suppliers and the combination of 14 components (including eight sourced from outside and developed specifically for the Pixie);
thermochromic printing;
assembly with a camera vision system control and pressure testing;
laser printing; and
skin applicator design.
Following prototyping, six injection molds were built for high-series production and the assembly process was finalized. Assembly involves several semi-automated quality control checks done under medical-device-regulated conditions and numerous automated quality checks.
The Pixie pen is CE marked and approved for use in the European Union as a Class IIa medical device. All processes at Oystershell and Quadrant have been accredited through external audits under standards equivalent to ISO 13485. Biological and clinical studies indicate that the Pixie pen is superior to various other available methods for the treatment of warts, according to the companies.
A key feature of the device is the tip of the applicator, which is made of polyurethane foam. The gas flows through this foam and freezes it. The user places the tip onto the wart, transferring the cold to the wart. The soft foam ensures that contact with the wart is ideal because when it heats up it makes excellent surface contact.
“The Pixie pen embodies the fusion of our knowledge in the field of plastics engineering and polymer processing and the knowledge of an external industry expert, in this case in the field of medical devices,” said Jan Claeys, Account Manager at Quadrant CMS.
About the Author(s)
You May Also Like