As medical device OEMs reshore some of their contract molding to minimize supply chain risks exposed by the pandemic, the custom injection molder is positioning itself as a reliable, growth-oriented partner.
June 22, 2020
Last month, PlasticsToday reported on how Sussex IM plans to cater to existing and potential customers seeking to reduce their supply chain risks by reshoring some of their activities. The full-service custom injection molder announced a plant expansion, integration of Industry 4.0 technology, and the addition of a Class 8 cleanroom at the time. Sussex IM released additional details about the cleanroom today.
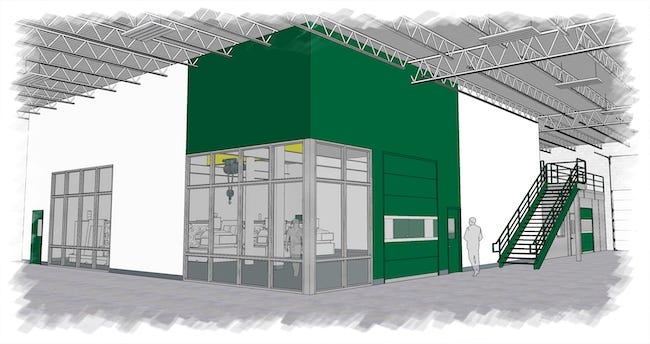
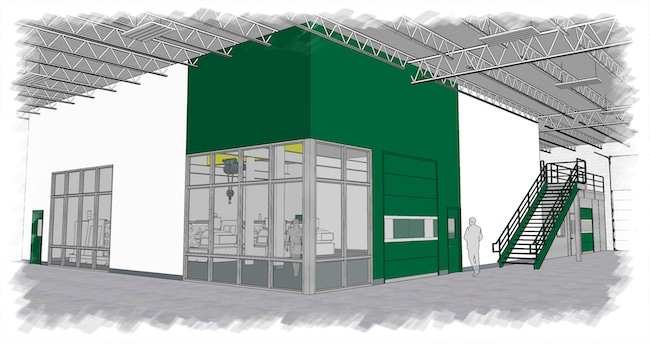
The ISO Class 8 cleanroom is expected to be completed later this year. A $2 million investment, the cleanroom is a further indication of its commitment to serving the growing medical market, said Sussex IM, which achieved ISO 13485 certification in 2018.
|
Rendering of new cleanroom provided by Sussex IM. |
The cleanroom is a critical expansion to Sussex IM’s existing capabilities, said Greg Gierach, Business Development and Sales Account Manager. “Our legacy of innovative automated manufacturing and improved facilities, coupled with the realities of COVID-19's impact to the global market, present a unique opportunity. Procurement practices in all segments have quickly adapted and will continue to evolve. We believe this will be amplified in the medical, pharma, and hygiene segments. The addition of this cleanroom capacity will benefit our existing customers and position Sussex IM for growth with new strategic partners,” said Gierach.
Throughout the pandemic, Sussex IM has remained completely operational and set its sights on preparing for life after COVID-19, said the company. Being flexible and adapting to changing circumstances is more important than ever, said Sussex IM, adding that it is actively planning to adapt to the likelihood of OEMs quickly increasing reshoring efforts in the coming months.
The new cleanroom is part of a multi-year, phased approach to continue to add flexibility and increase capacity to serve the medical market.
Describing itself as a “reshore enabler,” Sussex IM said that it will mitigate variables in transportation costs, lead times, duties, and tariffs. “The phased growth strategy will allow us to tailor equipment and process flow, reacting according to this changing landscape,” said Gierach.
“We see issues in the complicated global supply chain that have created a demand for more medical molding cleanroom capacity,” added CEO Keith Everson. “This is the first step of many we are taking to solve these problems.”
In addition to ISO 13485, Sussex IM is certified to ISO 14001, ISO 9001, and ISO 45001.
About the Author(s)
You May Also Like