Thermoforming and pressure forming offer medical device OEMs an alternative to injection molding for the production of a range of plastic enclosures.
Hayley Haggarty
From changing customer demands and market shifts to new technologies, regulatory pressures, and more, medical device manufacturers have always navigated a complicated environment. Add a global pandemic to the mix, and such manufacturers are now facing mounting pressure to develop increasingly complex products and rapidly bring them to market. As a result, an integral element of the manufacturing process is undergoing increased scrutiny to ensure medical device packaging and enclosures adhere to changing FDA and ISO 11607 regulations, the recognized guidelines for validating terminally sterilized medical device packaging.
To meet the rigorous requirements of medical devices and to arm healthcare workers with products that are safe, sterile, and ready to use in the battle against COVID-19 and other medical conditions, many manufacturers are turning to thermoformed and pressure-formed solutions for product packaging. Such processes are experiencing more usage due to their versatility, accurate part-to-part repeatability, and low production cost.
To spotlight the critical integration of thermoforming and pressure forming in the global supply chain, Informa Markets – Engineering, the organizers of the industry-leading Medical Design & Manufacturing (MD&M) and BIOMEDevice events and publisher of PlasticsToday, will present MD&M | BIOMEDigital, a new virtual conference and exhibition for medical device engineers and manufacturers driving progress and innovation in medtech and biotechnology.
Taking place April 6 and 7, the virtual event will provide a unique meeting opportunity for the global community featuring a virtual expo floor with more than 100 companies that are driving frontline product innovation. The conference will include a robust agenda offering technical sessions curated around today’s challenges, from integrating new materials and technologies into the manufacturing process to showcasing innovations in micro-molding medical devices.
Register here and access exhibitor profiles, exclusive networking opportunities, and technical sessions that matter to you.
I had the pleasure of speaking with Jason Middleton, VP of sales and development at Ray Products, a custom heavy-gauge thermoforming manufacturer that will be exhibiting at MD&M | BIOMEDigital, to discuss the importance of the upcoming events and what attendees can look forward to learning at the company’s virtual booth.
|
Jason Middleton, VP of sales and development, Ray Products. |
Ray Products has a rich history in the development of thermoformed parts and has transformed into a cutting-edge company that develops custom plastic parts for advanced machinery and materials for a widening variety of industries. How does your suite of products serve the medical field?
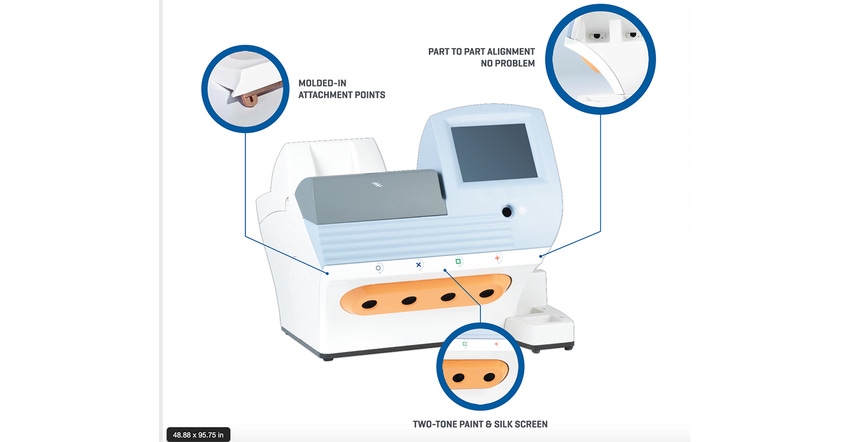
Jason Middleton: In the medical product market, there is a critical need for part repeatability, with each produced part being subject to stringent requirements such as UL 94 V0 flame rated, FDA approved, and chemical- and impact-resistant, to name a few examples. Thermoforming/pressure forming is perfectly suited for the medical device industry because it satisfies the demand for a variety of plastic enclosures. At Ray Products, our capabilities allow us to provide attractive and durable solutions that offer an alternative to injection molding.
As medical devices become progressively complex and assembly requires increasingly custom parts, how is Ray Products continuing to drive innovation and a more effective end product?
Middleton: Technology and innovation are what drive us. Utilizing the latest pressure forming equipment, including six-axis robotic trim centers for secondary operations, Ray Products can meet the needs and complexities required by our customers. To do this, we add capabilities for assemblies like sheet metal to our plastic parts to help cut down the time for our OEMs at their assembly line.
As a first-time and prominent exhibitor at MD&M | BIOMEDigital, what suite of products and services can attendees look forward to learning about at your booth?
Middleton: We will be available in the booth to answer any design or process-specific questions, helping attendees explore the versatile capabilities of pressure forming. As experts, Ray Products can help you discover the best fit for your manufacturing project.
When you visit us, you will discover several case studies showing where our company (and the process) have been highly successful within the medical device space. Our virtual booth will also feature our Design Handbook – a must-have for anyone new to pressure forming or anyone who wants to receive a profound understanding of what the process can achieve.
What excites you most about connecting with your community at the upcoming virtual event?
Middleton: We are a very engaged company. We have always found creative and unique ways to connect with the medical device community, either at a trade show, a lunch and learn, a facility tour, a webinar, or a design review via Zoom. MD&M | BIOMEDigital is another unique way to be social and directly engage the medical device industry.
How does Ray Products plan to leverage the industry-leading virtual event to further safeguard patients and medical professionals?
Both the medical device and biomed markets are extremely high tech and automated, so it makes sense that Informa Markets – Engineering, organizers of MD&M | BIOMEDigital, are using a high-tech, digital platform to bring the industry and supply chain community together in one event, allowing all to continue to support each other with industry-leading solutions. We look forward to showcasing our process capabilities and how they can benefit the medical market.
To schedule a meeting with Ray Products at MD&M | BIOMEDigital, please register for the event here and reach out to Jason Middleton, VP of sales and development, at [email protected].
About the author
Hayley Haggarty is Group Event Director, MD&M | BIOMEDigital, at Informa Markets – Engineering.
About the Author(s)
You May Also Like





