March 17, 2021
FDA has issued a safety alert in light of a “higher than expected risk” of a plastic component breaking in an ankle-replacement device made by Stryker. Fracture of the polyethylene-based part in the Scandinavian Total Ankle Replacement (STAR Ankle) device could occur as early as three to four years after implantation, said FDA in its alert.
Based on recent analysis of post-approval studies and adverse event reports, the agency stressed that risk of the component breaking “may exist for all STAR Ankle devices, regardless of the date of manufacture or distribution.”
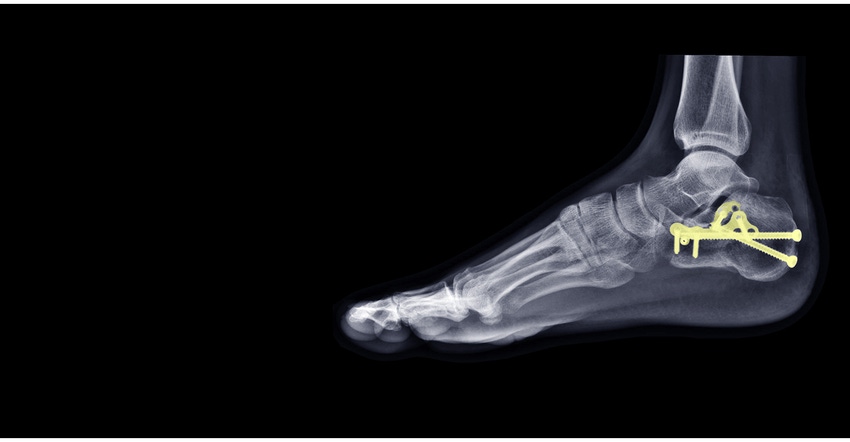
The STAR Ankle was one of the first flexible implants designed to replace ankle joints, reports Fierce Biotech. It is used to replace a painful arthritic ankle joint caused by osteoarthritis, post-traumatic arthritis, or rheumatoid arthritis, notes FDA in its alert. The STAR Ankle comprises a tibial plate, a mobile-bearing polyethylene component, and a talar component, according to FDA, and is designed to allow for some normal ankle mobility and function. The mobile-bearing device component is made from sterilized polyethylene.
Based on a long-term post-approval study, FDA found that the plastic component fractured at a cumulative rate of 13.8% eight years after implantation. The agency also noted that fractures were observed as early as three to four years after implantation. The combination of a high fracture rate and earlier than expected occurrence are concerning when compared with other total ankle replacement devices, according to FDA.
The agency has previously raised concerns about the long-term strength of the polyethylene plastic component, and Stryker made changes in 2014 to its pre-implantation packaging to better protect the pieces from degradation, reports Fierce Biotech. “However, data from post-approval studies and real-world monitoring showed unexpectedly high rates of plastic fractures, regardless of the packaging or manufacturing date — potentially due in part to the artificial ankle’s use in younger populations of more active people,” writes Conor Hale.
FDA notes in its alert that it reviewed data provided by the manufacturer on 244 STAR Ankle implants that were removed (including devices manufactured after the 2014 packaging change), which showed 72 plastic component fractures. Fractures were observed more frequently in 6-mm-thick plastic device components as opposed to thicker 7- to 9-mm components. Only one fracture was observed in the 11- to 14-mm thickness range. “Most of the plastic component fractures showed material oxidation degradation after three to four years of implantation and exhibited loss of mechanical properties,” said FDA.
The fractures may be attributed to multiple factors, added FDA, including device design (component thickness), material degradation, surgical factors, and patient factors.
FDA believes that the STAR Ankle remains appropriate for older patients with lower activity levels. The agency cautions, however, that patients with more active lifestyles, who are younger than 55, or who suffer from osteoarthritis may have a higher than expected risk of the plastic part breaking.
About the Author(s)
You May Also Like




