A new line of custom PEEK-based skull implants, individually tailored to a patient’s cranium on the basis of CT scans, is coming to market after receiving Food and Drug Administration (FDA) approval in April.
July 6, 2011
A new line of custom PEEK-based skull implants, individually tailored to a patient’s cranium on the basis of CT scans, is coming to market after receiving Food and Drug Administration (FDA) approval in April. Kelyniam Global Inc. (Canton, CT) received FDA 510(k) clearance to market the cranial implants on April 20, with the devices utilizing Invibio’s Optima brand polyetheretherketone (PEEK).
Kelyniam said its Custom Skull Implants (CSI) rely on the strength, bone-like modulus, radiolucency, and purity of Invibio’s Optima material, which is biocompatible and has seen use in implants since 1999 with almost 3 million implanted devices in use.
James Ketner, Kelyniam CEO, told PlasticsToday that using CAD/CAM design software enables Kelyniam to deliver patient-specific implants in as little as 24 hours from the receipt of CT scans, shortening the time between a head trauma and implantation. Such implants are currently sourced from cast acrylic, which requires a one-off mold and take four to eight weeks to produce.
With Kelyniam’s product, a surgeon receives an SLA “host bone” which recreates the area of the skull where the implant will go, allowing them to test form, fit, and function prior to the operation while also locating the best attachment points, and the implant itself, which is machined from Invibio’s Optima PEEK.
The company says a wide range of commercially available fixation systems can be used to attach the Kelyniam CSI to native bone, allowing surgeons to use their preferred fixation device. The customized design of the skull implants allows operating room time to implant the devices to be cut by as much as 85%, according to the American Journal of Engineering and Applied Sciences.
Kelyniam’s CSIs are designed to correct or replace bony voids in the cranial skeleton caused by trauma or birth defects. The company began development of the CSI product in the second quarter of 2009, and in less than two years, it says it has perfected a process to quickly and accurately deliver high-tolerance patient-specific custom skull implants.
Kelyniam also plans to develop and in-license additional medical devices for surgical markets, with these products marketed through independent sales representatives. In March 2010, Kelyniam signed a supply agreement with Invibio wherein all of its maxillofacial and custom cranial implants will be made with Invibio’s PEEK-Optima polymer.
Ketner said that upon receiving FDA approval for the device, his company was inundated with interest; enough so that it immediately began construction of a new, larger facility. The company has already moved into the ISO 13485 designated site located in Canton, CT, and the current plan calls for commercial production of the implants, in 5000-sq-ft of manufacturing space, to begin at the end of July.
From a capacity standpoint, Ketner said Kelyniam has purchased an additional 5-axis CNC machining center and doubled its number of stereolithography (SLA) 3D-printing systems from four to eight. Ketner believes that over the next six months, Kelyniam’s headcount will also double from its current 14. The technology holds additional promise, with Ketner noting that his company will file for another 510K in the third quarter on an undisclosed device.
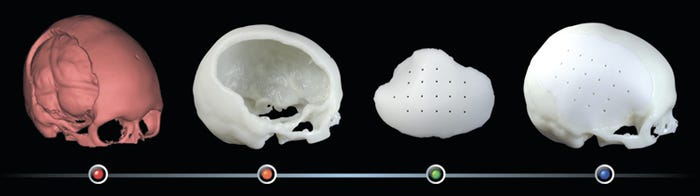
Kelyniam's PEEK Optima custom skull implant.
About the Author(s)
You May Also Like