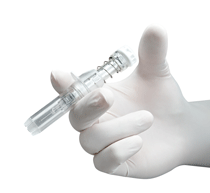
The U.S. Food and Drug Administration granted 510(k) approval for Safe'n'Sound, a passive safety device for staked prefilled syringes. Rexam Healthcare is the processor of these devices.
September 7, 2011
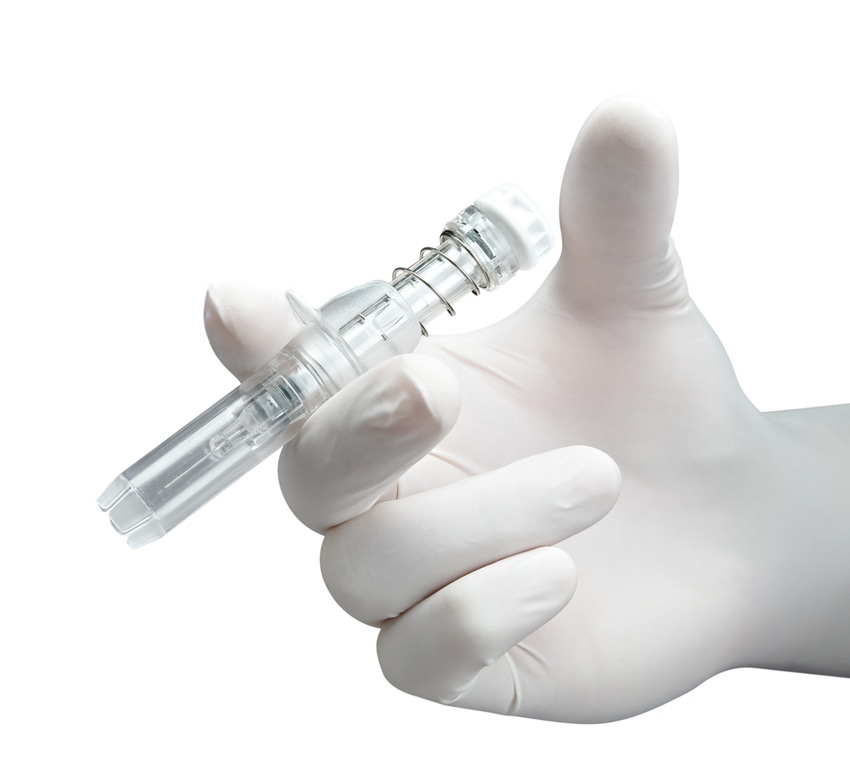
The U.S. Food and Drug Administration granted 510(k) approval for Safe'n'Sound, a passive safety device for staked prefilled syringes. Rexam Healthcare is the processor of these devices.
|
With the Safe'n'Sound device, Rexam says its goal was to design a safety device that meets the current regulations in North America and in Europe aimed at protecting workers in the health sector from needle injuries and contamination from blood-borne pathogens. The devices are patented and the entire needle plus sheath is made from just four components.
The fully passive Safe'n'Sound device provides protection against the risks of being pricked by a soiled needle, as the device comes with a protective sheath which activates automatically once the medicine has been administered.
This 510(k) approval allows Rexam to market in the United States.
About the Author(s)
You May Also Like