Chalk up another victory for polyetheretherketone (PEEK) in the metal replacement sweepstakes. Thompson MIS (Salem, NH), a supplier of minimally invasive spinal implants and delivery systems, has received 510(k) clearance from FDA for its BoneBac TLIF spacer made of Zeniva PEEK resin from Solvay Specialty Polymers (Alpharetta, GA). Solvay made the announcement at the North American Spine Society's annual meeting last week in San Francisco.
November 17, 2014
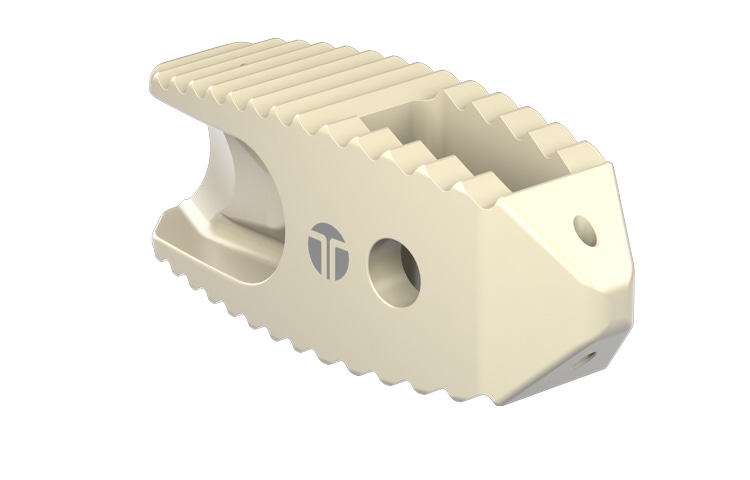
Chalk up another victory for polyetheretherketone (PEEK) in the metal replacement sweepstakes. Thompson MIS (Salem, NH), a supplier of minimally invasive spinal implants and delivery systems, has received 510(k) clearance from FDA for its BoneBac TLIF spacer made of Zeniva PEEK resin from Solvay Specialty Polymers (Alpharetta, GA). Solvay made the announcement at the North American Spine Society's annual meeting last week in San Francisco.
Part of Solvay's Solviva line of biomaterials, Zeniva PEEK has a modulus very close to that of bone plus excellent toughness and fatigue resistance. The FDA clearance was based, in part, on Solvay's master access file for the material, according to the company.
 The BoneBac TLIF spacer is a transforaminal lumbar interbody fusion spacer. These spacers are implanted in the lumbar disc space to enable fusion of the adjacent bony surfaces of the vertebrae. Minimally invasive surgery is made easier with this spacer thanks to its patented geometry that allows for direct injection of graft while the inserter is still attached, resulting in a more complete fill when packing the disc space. The BoneBac TLIF spacer also provides surgeons with two options for implant insertion.
The BoneBac TLIF spacer is a transforaminal lumbar interbody fusion spacer. These spacers are implanted in the lumbar disc space to enable fusion of the adjacent bony surfaces of the vertebrae. Minimally invasive surgery is made easier with this spacer thanks to its patented geometry that allows for direct injection of graft while the inserter is still attached, resulting in a more complete fill when packing the disc space. The BoneBac TLIF spacer also provides surgeons with two options for implant insertion.
Zeniva PEEK is a comparable alternative to metals such as titanium for these intervertebral implantable devices, according to Thompson MIS. The material offers many important benefits including biocompatibility and chemical inertness. Based on biocompatibility testing, Zeniva PEEK demonstrates no evidence of cytotoxicity, sensitization, irritation, or acute systemic toxicity. The material meets the ASTM F2026 standard, which provides the requirements and associated test methods for PEEK in virgin form used in the manufacture of intracorporeal devices. The material also boasts high strength and stiffness and has radiolucent properties that enable x-ray procedures without interference.
Thompson MIS uses Zeniva PEEK rod stock and performs high-precision machining to produce a full range of sizes and configurations.
"We're excited about the commercial success of Zeniva PEEK in the spinal fusion market," said Shawn Shorrock, Global Director of Regulatory Affairs for Solvay Specialty Polymers' healthcare business in a prepared statement. "The ongoing acceptance of Zeniva PEEK has validated our approach to the spinal market, and we're encouraged by the momentum we've generated."
The manufacturing site for Zeniva PEEK and other Solviva biomaterials in Alpharetta is certified to ISO 13485, the international standard for quality systems in the design and manufacture of medical devices, and follows current Good Manufacturing Practices. Solvay's biomaterial manufacturing processes are carefully validated and enhanced controls provide product traceability. In addition, all materials are tested in an ISO 17025 accredited lab.
All of Solvay's Solviva biomaterials are available in injection molding and extrusion grades as well as rods and plates for machined components.
About the Author(s)
You May Also Like


