The U.S House on Monday swiftly passed a bill to ban plastic microbeads in over-the-counter (OTC) and personal-care products, starting in 2017. The legislation, which is headed next to the Senate for review, would ban the manufacture of microbeads beginning in 2017 and eliminate their use in cosmetics and over-the-counter drugs in 2018 and 2019, respectively. The bill cleared the House unanimously after previously passing the House Energy and Commerce Committee, also without objection, in November.
December 8, 2015
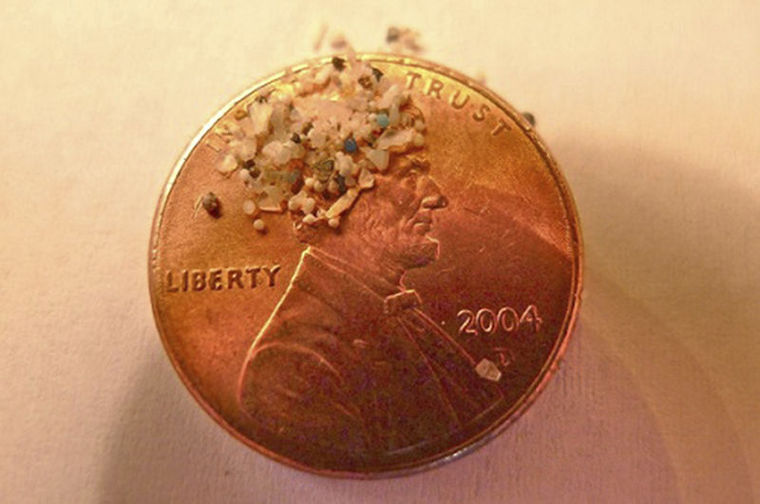
 Plastic microbeads have been in the middle of a heated debate as concern has been raised among clean water advocates on the challenging removal process typically done in wastewater treatment processes. The microbeads are able to crossover into the waterways during these, inflicting harm to marine life. Currently, there are other natural alternatives available to replace plastic microbeads in personal care and OTC products.
Plastic microbeads have been in the middle of a heated debate as concern has been raised among clean water advocates on the challenging removal process typically done in wastewater treatment processes. The microbeads are able to crossover into the waterways during these, inflicting harm to marine life. Currently, there are other natural alternatives available to replace plastic microbeads in personal care and OTC products.
Consumer Healthcare Products Association (CHPA) President and CEO Scott Melville released the following statement commending the U.S. House of Representatives for speedy passage of the Microbead-Free Waters Act of 2015 (H.R. 1321). The bill, sponsored by U.S. Rep. Frank Pallone, Jr. (D-NJ) and U.S. Rep. Fred Upton (R-MI), provides a reasonable timeframe for the removal of synthetic plastic microbeads from over-the-counter (OTC) and personal care products.
“The OTC medicine industry applauds Representatives Pallone and Upton for shepherding legislation that sets forth feasible phase-out dates for plastic microbeads in OTC products, appropriate definitions of key terms, and ensuring uniform enforcement across the nation.
“Our industry takes concerns about these solid plastic microbeads possibly entering the marine environment very seriously, and we are committed to reformulating cosmetic OTCs—such as acne face washes and toothpastes—to remove plastic microbeads. CHPA member companies have already taken steps to voluntarily remove these solid plastic microbeads from their products by ceasing the development of any new products containing synthetic plastic microbeads and working toward formulating replacement products. The timeframe allocated in this bill provides manufacturers adequate time to identify and phase in alternatives.”
The American Chemistry Council (ACC) also praised lawmakers for passing the legislation on Monday.
“Plastics play a vital role in our economy—from helping build and maintain homes to advancing new technologies,” the industry group said. "H.R. 1321 is an important step to ensure we have one sensible, national standard for phasing out the use of solid plastic microbeads in personal care products across America."
CHPA along with the ACC, Personal Care Products Association, and SPI: The Plastics Industry Trade Association sent a letter of support for the legislation to Reps. Upton and Pallone on Nov. 17.
About the Author(s)
You May Also Like


