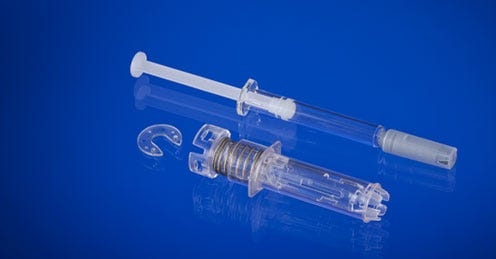
West boosts molding capacity in France for safety syringe
West Pharmaceutical Services (Lionville, PA) is adding significant molding and assembly capacity in France to produce its new B.safe syringe safety system, which is designed to help protect against needlestick injuries when using a prefilled syringe.The large investments are being made at West's Normandie facility, located in Le Vaudreuil, France, which has produced more than 1 billion units of the company's eris safety system. Significant scale-up to high-volume manufacturing is expected in the first half of next year.
September 25, 2012
West Pharmaceutical Services (Lionville, PA) is adding significant molding and assembly capacity in France to produce its new B.safe syringe safety system, which is designed to help protect against needlestick injuries when using a prefilled syringe.
The large investments are being made at West's Normandie facility, located in Le Vaudreuil, France, which has produced more than 1 billion units of the company's eris safety system. Significant scale-up to high-volume manufacturing is expected in the first half of next year.
|
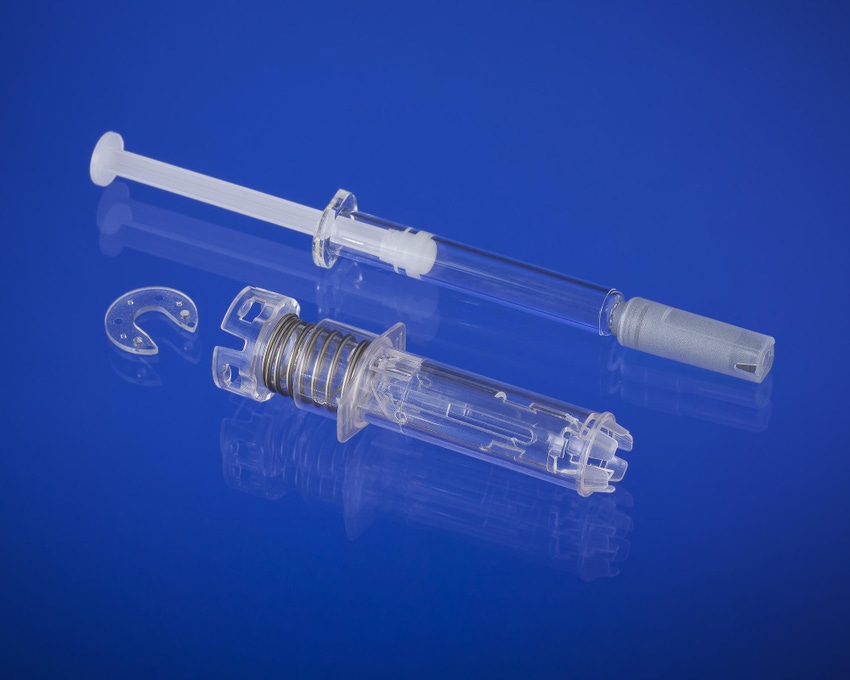
New system targets syringe accidents. |
In 2011, West acquired the IP and rights to the B.safe system, which will fit into the company's portfolio of prefilled syringe offerings and technologies, including the NovaGuardT passive needle system for Luer Lock syringes and the érisT system.
B.safe is a registered trademark of Tech Group Europe.
"We are delighted to reach this significant milestone and be in a position to offer our customers a globally available safety system, leveraging our established expertise around prefilled syringes and needle safety," said Glenn Thorpe, VP, Strategic Market Development, Syringe Systems. "With the completion of the steps necessary to offer this system commercially, we are in position to meet growing market demand in this area, and are pleased to have already signed our first commercial agreement and commenced shipments."
The B.safe system is compatible with a range of ISO-compliant prefilled syringes, including glass and polymers. In some cases, it is manufactured with a Crystal Zenith (CZ) pre-fillable syringe. "CZ is a novel material made from cyclic olefin polymers, which are more break-resistant and inert than glass," a company spokesman told PlasticsToday. The CZ prefillable syringes were first developed by West Pharma's Japanese partner, Daikyo Seiko, to counter breakage problems with glass syringes.
Designed for syringes with a rigid needle shield, the new system comes in 0.5 -ml and 1-ml versions.
The Tech Group was established in Phoenix, AZ in 1967 by Steve Uhlmann and was acquired by West Pharmaceutical Services in 2005.
You May Also Like