Sterilization requirements help drive Ultem adoption
Sabic Innovative Plastics says independent third-party testing of its Ultem polyetherimide (PEI) material shows it can stand up to gas plasma sterilization, an increasingly popular method of disinfecting medical devices and components known commercially as Sterrad. Patented in 1987 and introduced in the U.S.
June 29, 2011
Sabic Innovative Plastics says independent third-party testing of its Ultem polyetherimide (PEI) material shows it can stand up to gas plasma sterilization, an increasingly popular method of disinfecting medical devices and components known commercially as Sterrad. Patented in 1987 and introduced in the U.S. in 1993, Sterrad was initially applied by large manufacturers of end-of-the-line in-house sterilization, according to a Centers for Disease Control report. Now the Ethicon/Johnson & Johnson technology is available in more than 60 countries, with 6000-plus units sold, making it the fastest growing sterilization technology, according to the CDC.
"[Sterrad] has been around for a few years, but it's one that we're hearing more and more about it," Tom O'Brien, Sabic's global healthcare specialist, explained to PlasticsToday. "As the devices get smaller, more delicate, they still need to be sterilized, but OEMs are starting to look for techniques that are a little less aggressive for more delicate type instruments."
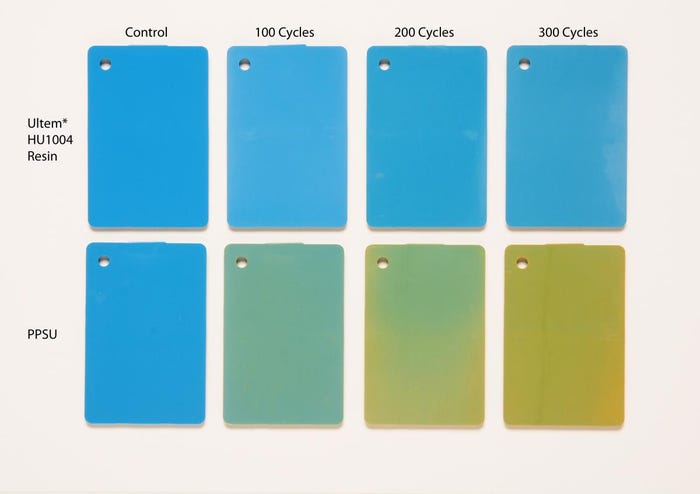
Sabic's study compared its Ultem (PEI) HU1004 resin to polyphenylsulfone (PPSU), showing that over time, the Ultem showed "significant performance and aesthetic advantages" over the sulfone material, particularly in its ability to maintain ductility and color stability.
Sabic believes the material's strong performance after repeated cycles of the low-temperature hydrogen-peroxide-gas plasma sterilization allows healthcare providers to choose any major method for sterilizing trays, electronic medical devices, and other applications.
Trial by plasma
To validate the mechanical properties and degree of color shift for Ultem HU1004 resin after exposure to the Sterrad NX method, Sabic Innovative Plastics commissioned independent testing lab SPS Medical (Rush, NY), which exposed samples of Ultem resin and PPSU to Sterrad NX sterilization in increments of 50 cycles going up to 300. These samples were then evaluated by Sabic's technical experts.
Sabic Ultem steriization test |
This test shows color fade in blue, white, and gray samples. |
Sabic Ultem steriization test |
This shows color fade in Ultem and PPSU after 100, 200, and 300 cycles. |
The findings showed that PPSU exhibited a color shift up to 10 times greater than Ultem HU1004 resin, becoming significantly yellowed after 100 Sterrad NX cycles, while Ultem also better retained mass and impact performance. Even without an impact to the materials' mechanical properties, retention of color is a key attribute that goes beyond visual appearance.
"Aesthetics is definitely a key thing," O'Brien said. "If you're a surgeon, and it's a surgical device or a tray, you're never going to want to see the color shift. Being in this business, we look at the part and say, 'OK, there may or may not be something wrong with the material, with the mechanicals,' but to a doctor or to a nurse, they don't understand that, and they think something's wrong."
Weight Loss (after 150 and 300 Sterrad NX cycles)
Ultem HU1004 resin lost less than .5% of its weight after 150 and 300 Sterrad NX cycles
PPSU had a 2.25% weight loss after 150 Sterrad NX cycles and a 7.7% loss after 300 cycles.
Tensile Properties Retention (after 150 and 300 Sterrad NX cycles)
Ultem HU1004 resin retained more than 99% of its tensile strength at 150 cycles and 98% at 300 cycles. For tensile elongation at break, Ultem retained 94% of performance at 150 cycles and 77% at 300 cycles.
PPSU retained 96% of tensile strength at 150 cycles and 91% at 300 cycles. The material became brittle and retained only 12% of its elongation at 150 cycles, and 11% at 300 cycles.
Sabic calls its Ultem HU1004 "an excellent candidate material" for healthcare applications requiring high temperature autoclaving, ethylene oxide, gamma radiation and low-temperature hydrogen peroxide gas plasma sterilization processes.
Market pull
O'Brien said the initial impetus for the development came from a customer that wanted to be able to use its device in multiple sterilization methods. "What it provides the OEM the ability to do is instead of saying, 'OK here's one device that's going to fit people that use Sterrad; here's another device that's going to use high-heat autoclave,'" O'Brien said, "I can now deliver one device, and it's going to cover all four of the major sterilization methods."
In addition to being able to satisfy more methods, O'Brien said materials are being asked to endure the processes at a greater frequency. He noted that Ultem can now go out to 2500 autoclave cycles at 134°C and still show no change in ductility, while also being able to endure more aggressive chemical environments being deployed to combat infections. Above all, the faster healthcare providers can get their equipment back in service, the faster they can treat patients.
"Speed is key," O'Brien said, "because the faster they can sterilize, the faster that device can be put back into use. But also, they're making sure they're getting good sterilization and they're not damaging the devices. Sterilization is one of the things that customers and OEMs are looking at more and more, and considering what type do I need, for how long, and what's going to give me the maximum sterilization at the lowest overall cost."
To that point, on its website, Advanced Sterilization Products (Irvine, CA), which sells the technology as a division of J&J, bills Sterrad as offering "convenient, quick turnaround of operating-room-ready instruments" with as low as a 28-minute cycle time allowing more caseloads.
About the Author(s)
You May Also Like