A User-Friendly Guide to Medical Plastics Selection
There are hundreds of thousands of plastics on the market today. In part one of this two-part article, plastics engineering expert and industrial designer Michael Paloian explains how to identify the optimal plastic resin for a given medical application.
March 19, 2021
Specifying plastics for medical devices can be a daunting task for anyone not familiar with the materials. There are hundreds of thousands of commercially available plastics on the market today, which is an overwhelming number of materials to sort through. This article is intended to narrow down your choices to a handful of viable materials based on critical criteria. We will highlight the interrelationships between plastic chemical structures and physical properties, and several other essential selection parameters.
Optimal plastics specifications for medical devices are essential due to compliance with numerous regulatory bodies and challenging physical properties. Some of these properties include extremes in temperature, stiffness, tensile strength, impact strength, and chemical resistance. Chemical resistance is especially critical, since very harsh disinfectants cause stress cracking or partial dissolution of plastics, depending on the material and reagent. Medical devices also must withstand long-term exposure to high temperatures, sometimes under high loads during sterilization. Medical devices are also required to withstand high temperatures, maintain structural integrity, and exhibit high impact strength. It is not easy to find plastics that exhibit high marks in all these properties, since high-temperature resistance and stiffness are often inversely proportional to impact strength for most plastics. Plastics used in medical devices also must retain properties for long periods of time and undergo extensive testing to verify that they can reliably perform for extended timeframes.
Low toxicity is another important consideration. Fillers, stabilizers, pigments, and additives all must be compliant with regulatory requirements for safety. Chemical leaching during thermal cycling, soaking, or other environmental conditions, which may promote the diffusion of additives into the surrounding media, must be minimal. During sterilization, selected plastics must withstand extreme conditions resulting from dry heat steam autoclaving, gamma radiation, ethylene oxide, electron beam, x-rays, ultraviolet light, and microwave energy. Medical devices are frequently sterilized with highly toxic gases, such as propylene oxide, chlorine dioxide, and ozone, which adversely affect certain plastics.
When you account for all these considerations, the previously mentioned massive list of hundreds of thousands of plastics quickly narrows down to a tiny group of candidate materials. For example, elastomers such as silicones and fluorocarbons are resistant to all the sterilization techniques shown in the table below. High-performance engineering plastics resistant to autoclaving, dry heat, ethylene oxide, gamma radiation, and electron beam sterilization include polysulfones, polyphenylene sulfide, liquid crystal polymers (LCPs) and polyetheretherketone (PEEK). How does one select the optimal plastic resin? The remainder of this article aims to provide you with the answer to this question.
The six fundamental parameters of plastics selection.
No one is aware of all the properties of every plastic that exists. However, some people are more qualified to select the optimal plastics for a given application because they possess a fundamental understanding of essential criteria. You should be aware of six fundamental parameters before you specify a plastic for your next medical device. They are listed below.
Understand polymer classifications
Thermoset
Thermoplastic
Crystalline
Semi-crystalline
Define application requirements — product specifications
Performance
Safety
Aesthetic
Assembly & Service
Define regulatory requirements — material related
Identify critical physical property requirements
Mechanical
Electrical
Flammability
Thermal
Long term
Chemical
Define business requirements
Material cost
Material availability
Lead time
Minimum order size
Seek outside assistance
Plastic material consultants
Publications (books, magazines, periodicals)
Internet resources
Molders
Plastic resin technical advisers
Polymers: A brief review
It isn't necessary to have a PhD in polymer chemistry, but a basic knowledge of polymers is highly beneficial when specifying a plastic. We can start by distinguishing the difference between plastic and polymer, which are often used interdependently. Plastic refers to the physical state of a material, while polymer refers to the material itself. Polymers become plastic, easily deformed with force when they are heated. Polymers are long-chain molecules based on a repeating unit called a "mer," thus the word polymer. These mer units are chained together in long strings, creating very high molecular-weight molecules. Comparing polyethylene with a molecular weight of up to one million to a simple compound like ferric oxide with a molecular weight of 231, one can readily appreciate that polyethylene's molecular weight is more than 4000 times greater than ferric oxide! These long polymer chains are linked to one another in one of three different configurations — linear, branched, and crosslinked.
Linear chained polymers can be compared to a flexible string of pearls, which is why they exhibit flexibility, good impact strength, and a degree of order that account for their semicrystalline structure (in some cases), imparting good chemical resistance. Examples of linear chained polymers include high-density polyethylene (HDPE), polytetrafluoroethylene (PTFE), and polyamides (nylons).
Charm bracelets provide an excellent model for branched chained polymers. Branched chained polymers include a backbone of mers with branches of molecules hanging off the chain like charms on a bracelet. These branches tend to contribute to a randomized relationship between the polymer chains, which yield amorphic structures. Examples include polycarbonate, low-density polyethylene, polypropylene, PVC, and ABS. Amorphic polymers tend to have lower chemical resistance than semicrystalline polymers.
The steel girders of a tall building under construction provide an excellent visual example of a crosslinked polymer structure. Crosslinked polymers differ from linear or branched polymers because of their chemical bonds. Crosslinked polymers chemically connect the long polymer chains with strong covalent bonds versus the latter, which are held together with much weaker Van der Waal forces. Crosslinked polymers are often referred to as thermoset plastics, meaning they cannot be remelted because of strong covalent bonds connecting polymer chains in a three-dimensional network. Crosslinked or thermoset polymers generally exhibit high heat resistance, excellent chemical resistance, and high rigidity. Common examples include phenolics, polyesters, epoxies, silicones, and polyurethanes.
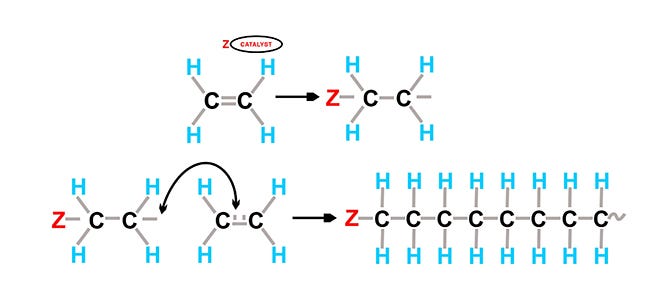
Two methods for polymerizing molecules are addition and condensation polymerization. Addition polymerization occurs when a monomer is added to itself thousands of times through some catalytic/thermal/pressure reaction. Examples of addition polymers include polyolefins, polystyrene, POM (acetal), ABS, and acrylics.
|
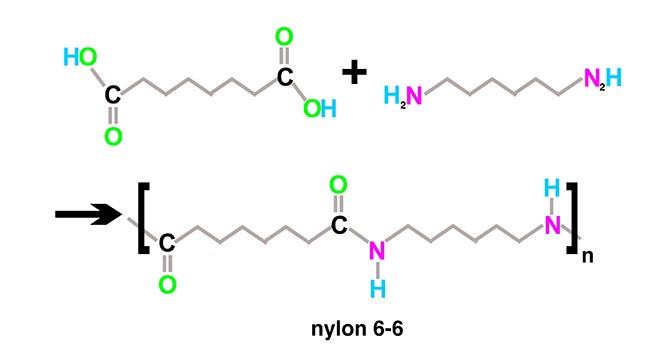
Condensation polymers are synthesized by reacting two chemicals that cause a chain reaction, adding identical pairs to the chain to form a polymer. This classification of polymers includes resins such as nylons, polycarbonates, polyurethanes, and epoxies. The name condensation polymer is derived from water as a byproduct of the reaction, making most condensation polymers susceptible to hydrolysis when exposed to water or steam.
|
Other unique classifications of polymers include elastomers, adhesives, copolymers, tripolymers, alloys, plastic fibers, and compounded materials. These alternative offerings create an almost infinite variety of options for plastics.
Chemical structure and physical properties of polymers.
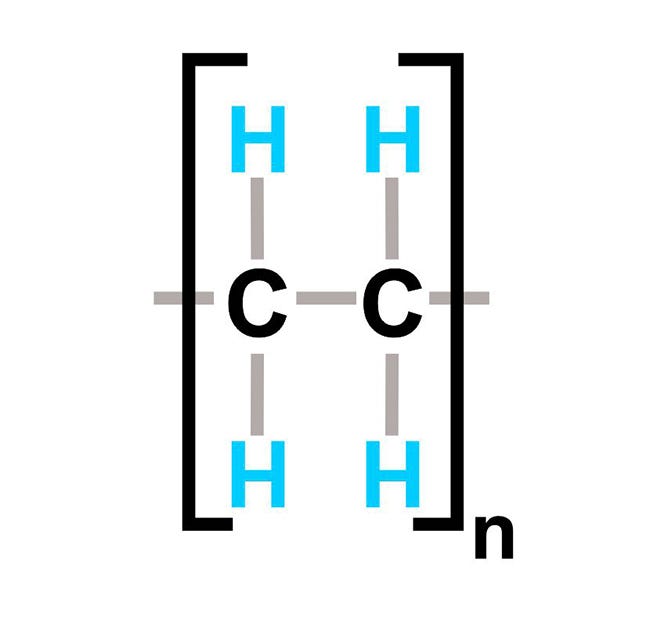
Our current basic understanding of polymer fundamentals provides us with a more in-depth look at the relationship between chemical structure and physical properties. Polyethylene is an excellent example of an addition polymer that is offered in many varieties, including linear low density (LLDPE), low density (LDPE), medium density(MDPE), high density (HDPE), crosslinked (XL PE), and ultrahigh molecular weight (UHMW PE). Although the polymer is based on the standard C2H4 mer unit shown below, the structure has many different configurations. These structural variations result in a variety of plastics with very distinctive properties.
|
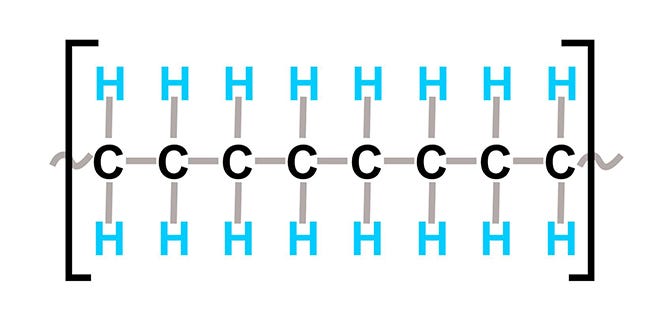
HDPE's chemical structure is shown below:
|
|
The linear chain of carbon and hydrogen atoms on either side of the carbons creates a non-polar molecule. The regularity of the chemical structure allows molecules to nest neatly, making a higher density material with an overall semicrystalline structure. These structural properties result in a material with the following physical properties when compared to LLDPE:
slippery feel
excellent chemical resistance
difficult to bond
marginal impact strength
high stiffness
high heat distortion temperature
high density
The LLDPE chemical structure is shown below:
|
Although LLDPE has the same formula as HDPE, the properties are quite different because of the difference in chemical structure. The properties of LLDPE are shown below:
slippery feel
moderate chemical resistance
difficult to bond
excellent impact strength
low stiffness — approximately 30% that of HDPE
low heat distortion temperature
low density
The physical property differences between LLDPE and PE are due to the polymer's branching, which contributes to a lower packing density of the molecules, thus lowering the density. Branching also diminishes crystallinity, which decreases chemical resistance. The lower molecular packing density contributes to the lower heat distortion temperature and lower stiffness.
When you compare polyethylene and polycarbonate chemical structures, the differences in physical properties become immediately apparent.
|
|
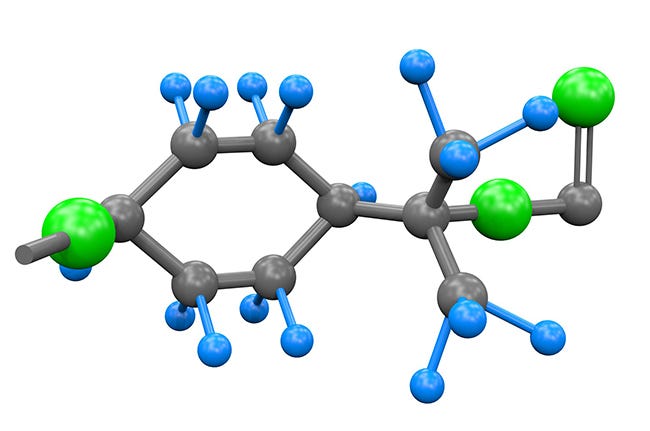
The polycarbonate mer is comprised of a carbon atom surrounded by three oxygen molecules and another carbon atom straddled by two methyl molecules, top and bottom, plus a pair of bulky phenyl molecules. When we analyze this chemical structure, it explains many of the properties inherent in polycarbonate. The oxygen molecules impart a high degree of polarity into the polymer, which is why polycarbonate is sticky during molding and is conveniently bonded with many solvents or adhesives. The larger phenyl rings are bulky, restricting molecular freedom of movement, thus imparting high stiffness and high heat distortion temperature. Carbon atoms in the polymer backbone provide a certain degree of flexibility, accounting for the material's superior impact strength. Polycarbonate's clarity is attributed to its amorphic structure. Since it’s a condensation polymer, polycarbonate is susceptible to hydrolysis, especially when exposed to steam.
A fundamental understanding of the correlation between the chemical structure and a polymer's physical properties will significantly reduce the guesswork required to identify the optimal plastic resin.
Application and product requirements.
As an engineer, you should know the application and product requirements, which are critical parameters for selecting materials. You should prepare a comprehensive product specification document outlining the product function and performance requirements. Even the slightest oversight defining product specifications could potentially lead to catastrophic problems. For example, parameters including assembly, misuse, environmental conditions, chemicals, and maintenance could significantly impact your material selection. Assembly might include adhesives, ultrasonic bonding, snap fits, tapes, press fits, or screws. All of these assembly methods are material-dependent. Bonding agents must be compatible with the plastic. Semicrystalline materials like POM (acetal, polyoxymethylene), nylon 6-6, polyethylene, polypropylene, and PTFE (polytetrafluoroethylene) cannot be bonded without special surface preparation. Other materials like polycarbonate will develop crazing or stress cracks if the adhesive is incompatible. Materials like polyethylene and PEEK are challenging to bond ultrasonically because of the former's non-polar chemical structure and the latter's high melting point.
Manufacturing requirements.
Plastic material choices are also dependent on the plastic molding process. Although most medical devices are injection molded, other methods such as thermoforming, composites, blow molding, and rotational molding are employed to manufacture numerous medical products. A smaller number of plastics are available for these other molding processes. For example, 90% of rotational molded parts are polyethylene; amorphic resins such as polystyrene, ABS, polycarbonate, acrylic, and PET are standard for thermoforming; while composites are limited to thermosetting resins like polyesters and epoxies. Medical devices such as MRI scanners, X-ray equipment, and PET scanners are typically manufactured by thermoforming or composites. Lower production volume medium-sized flat panels are sometimes blow molded in polycarbonate or polypropylene.
User requirements.
When you're specifying materials based on the user's requirements, your criteria may include ease of color matching, ease of pad printing, tactile feel, or clarity. The highly competitive medical device market has driven manufacturers to introduce attractive products. Product appearance is as essential to a product's acceptance as its function. Another user consideration is durability and a product's ability to withstand abuse. The technical specifications for all of these parameters must be clearly defined. Safety is a significant design consideration that requires a thorough investigation for potential mishaps, accidental misuse, mechanical failures, material failures, or failures caused by environmental conditions.
Regulatory requirements.
Unlike most consumer products, medical devices must comply with rigorous standards established by regulatory agencies within each country that profoundly affect material selection. Regulatory standards dictate specifications for properties such as impact strength during drop tests, self-extinguishing flammability ratings to prevent fire hazards, and high heat distortion temperatures for autoclaving. You may have numerous choices for plastics with high impact strength, but when you combine that with high-temperature resistance, your options will immediately narrow to a much smaller group. A further reduction in your selection will occur when you impose a 94-V0 flammability rating and UV resistance on your requirements list. High-voltage applications require materials to comply with specific insulation standards, which is a function of the dielectric constant. The ease of recycling is another significant consideration dictated by some regulatory bodies. Restrictions on traces of heavy metals such as chromium, lead, or cadmium could affect material stabilizers or pigments.
Physical property requirements.
Engineers select materials based on performance and functional requirements. The product specifications should clearly state these requirements. Typically, they are grouped into the following categories:
physical
mechanical
electrical/thermal
regulatory
long term
chemical/environmental
Although there are many readily accessible sources of information about plastic materials, most of the data are limited to standard properties and testing according to ASTM standards. An excellent data resource is MatWeb.com, which lists hundreds of thousands of plastics as well as other materials. If you're a registered user, you can enter multiple search criteria to identify one or more resins that comply with your specifications. This website provides a rapid and efficient method for narrowing down your selection to a small group of viable materials, but you, the engineer, must evaluate the options. These options must be assessed based on a clear understanding of the available properties and those that have been omitted. Resin manufacturers typically publish data that are favorable for their resins, often omitting negative information. This practice makes resin selection a complicated process. Some crucial physical property facts to consider when comparing resins to one another are listed below.
Although all material property data are tested according to ASTM standards, there are variations of many tests that complicate comparing one resin to another. A few examples are described below.
Impact testing. Plastic materials can be tested for impact strength based on notched Izod, unnotched Izod, Charpy, and drop ball impact. In addition, these tests can be conducted at room temperature or lower temperatures down to -40°C. Sometimes a 1/8-in.-thick specimen is used, and other times a ¼-in. sample is used. Comparing the performance of two materials tested under different conditions, therefore, is meaningless. All of these impact tests can only be used for comparative analyses since simulation software does not utilize the information. The impact strength of some materials like nylon 6 will change with moisture content. Dry nylon is much more brittle than nylon with a small percentage of moisture. Polycarbonate will not break during an Izod impact test but will fail when notched. Polycarbonate also exhibits much better impact strength in 1/8-in.-thick samples versus ¼-in.-thick specimens. The amount of butadiene in ABS will affect its impact strength. Higher butadiene content equates to higher impact strength at the expense of lower stiffness and heat distortion temperature.
Tensile strength and modulus of elasticity. Important physical properties for all applications are tensile strength and modulus of elasticity. So-called engineering plastics like nylons, polycarbonates, polyphenylene oxide, polysulfone, and polyetheretherimde exhibit a linear portion of their stress/strain curve, where stress and strain are directly proportional, thus providing a definitive Young's Modulus (tensile modulus). However, the majority of plastics don't share this property. The majority of polymers have a non-linear stress-strain curve, which results in a tensile modulus determined by a 0.2% tangent line of the stress-strain curve. Calculating deformation or stress requires non-linear finite element analysis (FEA). Also, the tensile properties of plastics are highly dependent on temperature. The rate of change of tensile strength as temperature increases varies based on the polymer. Amorphic plastics exhibit a more gradual change in tensile strength versus semicrystalline materials, which change more abruptly.
Temperature resistance. Defining the performance of plastic materials at elevated temperatures is extremely difficult to determine. Thermal properties for plastics are lumped together under HDT (heat distortion temperature) ASTM D648. This test determines the temperature that a supported sample beam deflects 0.01 when placed under a load of 264 or 66 psi. Some plastics exhibit much better HDT at 64 vs. 264 psi, therefore omitting the latter information. Since this is a relative test, the heat distortion temperature can only be used for reference. It would be a grave mistake to assume that the HDT represented the upper limit for a particular resin since the HDT is stress dependent.
Properties difficult to find. Unfortunately, most medical devices require material information that is not readily available anywhere. The following is an abbreviated list of some of the most common challenging types of data to search:
long-term chemical resistance
long-term creep resistance
fatigue resistance
wear resistance
There are many others. Each of these categories includes thousands of specific variations based on time, stress levels, temperature, rate/frequency, and concentrations. These tests are expensive to conduct and very time consuming, which is why they are not commonly available.
Medical devices are frequently molded from specially formulated resins that have been modified with additives such as reinforcements, fillers, special stabilizers, or even blended with other resins. These modifications change the physical properties, requiring the material to be thoroughly tested and recertified by regulatory agencies.
The distinctive property of all plastics is that they are viscoelastic versus elastic metals. Metals respond linearly to applied loads, returning to their original state after the load is removed. Plastic materials respond non-linearly to loads, exhibiting hysteresis after the load is removed. They do not always return to their original state. The term applied to this phenomenon is creep. Creep is dependent upon the applied load, time, and temperature. Creep, wear resistance, chemical resistance, and fatigue are fundamental long-term properties that must be understood for medical devices. Verification of material performance based on long periods will minimize the chances of product recalls. These polymers' unique characteristics complicate searching for the optimum resin and properly designing products based on the material's properties.
Read part two of this article.
About the author
Michael Paloian is President of Integrated Design Systems Inc. (IDS), located in Oyster Bay, New York. He has an undergraduate degree in plastics engineering from UMass Lowell and a master's of industrial design from Rhode Island School of Design. Paloian has an in-depth knowledge of designing parts in numerous processes and materials, including plastics, metals, and composites. Paloian holds more than 40 patents and was past chair of SPE RMD and PD3. He frequently speaks at SPE, SPI, ARM, MD&M, and IDSA conferences. He has also written hundreds of design-related articles for many publications. He can be reached by phone at 516/482-2181 or via e-mail, [email protected].
About the Author(s)
You May Also Like