NPE2015: Eastman, Prestige collaborate on multi-cavity mold for intricate medical parts
Calling the launch of Eastman's Tritan copolyester "one of the best polymer launches in the last 20 years," Burt Capel, Vice President and General Manager, Specialty Plastics, at Eastman Chemical (Kingsport, TN), went on to explain during a press conference at NPE2015 why the material continues to find new territories to conquer, specifically in the medical space. In collaboration with Prestige Mold Inc. (Rancho Cucamonga, CA), Eastman has developed what it says is a first-of-its kind multi-cavity hot runner mold for the production of intricate medical parts.
March 24, 2015

Calling the launch of Eastman's Tritan copolyester "one of the best polymer launches in the last 20 years," Burt Capel, Vice President and General Manager, Specialty Plastics, at Eastman Chemical (Kingsport, TN), went on to explain during a press conference at NPE2015 why the material continues to find new territories to conquer, specifically in the medical space. In collaboration with Prestige Mold Inc. (Rancho Cucamonga, CA), Eastman has developed what it says is a first-of-its kind multi-cavity hot runner mold for the production of intricate medical parts. The mold was developed to address anticipated customer needs for durable medical device parts free of bisphenols A and S that can withstand sterilization and harsh chemicals.
The merits of Tritan for medical applications are well known: in addition to chemical resistance, which is especially important as hospitals adopt harsher disinfectants to prevent hospital-acquired infections, the material has a low contamination rate and accommodates secondary processing well. To feature these qualities, Eastman and Prestige collaborated on building a mold that would work in harmony with the material. Mold-Masters contributed the hot-runner technology and Pres-Tek Plastics, a sister company of Prestige Mold that runs a medical molding and technology center with Class 8 cleanroom, provided mold validation.
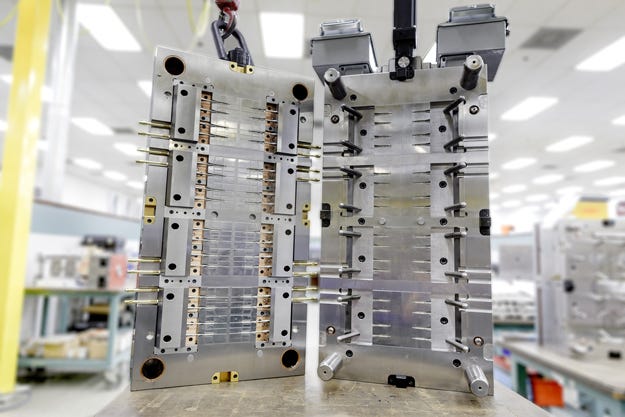
The demonstration multi-cavity mold was used to produce polished and unpolished universal female luers. Interestingly, the parts are designed to meet the forthcoming ISO 80369 standard for small bore connectors, which PlasticsToday reported on in an article titled, "International standard preventing medical device misconnections is-finally-on the horizon."
The outcome exceeded expectations, said Donna Pursell, Prestige Mold CEO, and Capel. The first run of the mold went perfectly, according to Pursell, with no process or ejection issues, even with the unpolished luers, which can stick to the mold. The 32-cavity mold produced equal numbers of polished and unpolished medical female luers.
"You typically expect some bugs in a custom project," added Pursell at the press conference. "But the early and ongoing collaboration led to a very successful conclusion. We all came together, relying on each other's expertise and pushing each other to succeed," said Pursell.
The demonstration mold is not intended for production, but in order to prove the concept and that production quantities can be met, the mold was built at production scale. In fact, NPE2015 attendees can see it in action at the Milacron booth (W-2703). After the show, the mold will be used globally for training purposes, said Capel.
To learn more about this collaborative success story, visit Eastman in room S-230D or Prestige and Press-Tek at booth W-6587.
About the Author(s)
You May Also Like




