Market Focus: Medical
March 1, 2001
| ||||||
From 1994 to 1998 the cost of health insurance grew more slowly than the overall rate of inflation and workers' earnings, according to Kaiser/HRET. However, increasing costs of technology and financial losses suffered by health insurers are now driving up rates. |
For the past few years the medical market has enjoyed a steadily escalating growth rate, and certainly as long as there are people, the health care industry will continue to exist. Still, inflation increases of the past few years (see table) portend a pattern of increasing costs. This in turn places a burden on manufacturers to reduce costs, yet still deliver a high-quality product. Furthermore, competition is getting stiffer with more international molders entering the market.
"There's a lot more competition in the medical device market than before. In the past the market seemed almost exclusive to the United States," says Chuck Brewer of C. Brewer Co. (Anaheim, CA). Brewer has taken notice of the increasing role Mexico is playing in medical injection molding. "Medical molding was not impacted dramatically by NAFTA [North American Free Trade Agreement]. However, now people are taking advantage of the low-cost labor."
One way his company is responding to global competition is through automation. "The more that we can automate and the more accommodating we are about embracing automation opportunities, the more competitive we're able to be in the global market," he says.
At Brewer's 55,000-sq-ft molding and moldmaking facility, five machines operate in a Class 100,000 cleanroom. "We're focused on precision molding applications, which is why we are not 100 percent cleanroom," he explains. "Some precision molding applications are molded in an open molding environment."
His forecast for the market's future lies in minimally invasive operations, such as laparoscopic surgery, where he sees a wealth of innovations taking place. "More procedures will be done that way, because there is less trauma on the patients and they're in the hospital less time. And as more doctors become proficient at it, the need for more plastic tools will increase. I think there's a lot of opportunity there," he says.
Mexico is not the only competition for those serving the medical industry. Cheryl Buhler, director of business development at AMA Plastics (Corona, CA), a medical disposables molder, sees Asia as a rising competitor. "Before it wasn't a big deal, because Asia didn't have a lot of cleanroom capabilities," she says. "However, during the last trip my management took there, we found a lot of molders putting in cleanrooms." In an effort to match the competition, AMA plans to expand into a 92,000-sq-ft building with designated areas for cleanroom molding.
Buhler also cites lengthy waits for material approvals by the FDA as a growing challenge. "Sometimes you don't get the resin that you want when you want it, or you can't get it at the price that you need to get it at, especially when the prices have been going up," she says. "It just makes it harder to be more proactive in cost reduction and things of that nature when you have to go through the whole approval process."
With a different view is Mark Schaefer, vp of business development at McKechnie Plastic Components in Minneapolis, MN. He sees the bright side of such obstacles. "We view quality, automation, and cost as the opportunity rather than the challenge," he says. "We are frequently converting materials to plastics, and by having the quality and the experience to automate, we are able to help our clients control costs while they expand and develop new products."
At the company's 100,000-sq-ft facility, the specialization is short- and long-term medical implants that use biomaterials. The molder's biggest challenge in the medical market is the unavailability of new biomaterials. "The market has not yet developed significant applications for injection molded uses of these materials," says Schaefer. He also believes that a shift from vertical integration to outsourcing assembly operations needs to take place for the market to take advantage its opportunities. "It's our belief that the medical market is poised to start adopting the information technology and automotive supply-based model," he says.
While the aging baby boomers and increasing technological advances will fuel the medical market over the next generation, some experts believe we may not see the numbers of the past few years.
"For the next year or two we are seeing steady growth, but not the 10 to 15 percent that has been historical," says Schaefer. "The marketplace isn't going to be growing much more—it's going to involve a relocation of manufacturing."
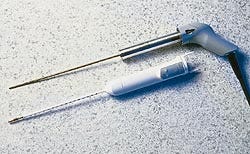 Two ABS grades meet tight design tolerances
Two ABS grades meet tight design tolerances
A new surgical device from CryoGen, designed to treat patients with abnormal uterine bleeding, required formidable material requirements—tight tolerances reaching as low as .0005 inch, radiation sterilization tolerance, excellent surface appearance, and competitive pricing. The answer proved to be Bayer's Lustran ABS, which is used in the pistol-grip handle and the disposable control unit.
Designed for intrauterine ablation (removal of tissue), the First Option Uterine Cryoblation Therapy system uses closed refrigeration to freeze tissue so it can be removed. While the system can produce coolant that reaches -120C, it is only the last inch of the probe tip that reaches these temperatures. The rest of the instrument remains at room temperature. This feature, along with its compact size and low operating pressure, allow the instrument to be used safely in the doctor's office for outpatient procedures.
The instrument is composed of four main components: the console that contains the refrigerant compressor; the flexible transport line that carries the coolant to the cryoprobe; the cryoprobe that is inserted into the uterus; and the control unit, a disposable component that slips over the probe.
The probe's three-part handle, molded with a gray Lustran 248 ABS, contains wall thicknesses ranging from .070 to .100 inch. In the front section of the handle, 12 pinholes requiring a tolerance of .0005 inch are designed to house spring-loaded contacts. The presence of the slightest draft may cause a contact not to be made, which will lead to the device's failure in the field.
"The Lustran material allows us to have low shrinkage, giving us the ability to handle these low tolerances," explains Steve Kovaicheck, senior project manager for CryoGen. "It also has a natural sheen so we didn't have to go to any lengths to polish the molds."
Plastics Engineering & Development (Carlsbad, CA) injection molds the three probe handle components in a family mold. Cycle times average 30 seconds.
The triangular-shaped control unit uses snow white Lustran 348 resin for the locking tab unit, shell, nose cone, and endcap. O-rings are used in the internal luer and fluid channels, and tolerances are ±.002 inch. Wall thickness is .100 inch. Florida Custom Mold injection molds the four components in single molds, averaging cycle times of 35 seconds for each component, and 60 seconds for the larger shell. The locking tab unit and nose are then bonded to the shell with adhesives and the endcap is snapfit to the nose.
Bayer Corp.
Pittsburgh, PA
Phone: (412) 777-2000
Web: www.bayer.com
 Five parts replaced with one
Five parts replaced with one
Five metal, steel, and plastic parts in the declutch tube of the P Series Syringe Pumps were replaced with a single injection molded component, reportedly lowering part costs by a factor of 20 to $1.10.
The electromechanical pump assembly from Alaris Medical Systems meters and dispenses drugs from a syringe to provide accurate drip feeding during intravenous therapy and in intensive care monitoring systems. The declutch tube, molded from Ticona's PBT, disengages the 5- to 100-ml syringes from the unit, enabling expended ones to be removed and fresh ones loaded.
Alaris selected the PBT, a 30 percent glass-fiber-reinforced grade, because it resists hospital cleaning chemicals and does not stress crack under long-term contact with O-ring lubricants. It is also rigid enough to withstand the torsional loads involved, has high impact strength and dimensional stability, meets UL94 V-0 flammability standards, offers a smooth surface finish, and can be retrofitted to existing units by ultrasonic welding.
The 173-mm-long declutch tube is molded around a core pin, and Ticona performed flow analyses to help ensure that the cavity would fill properly. This involved evaluations of the melt front, temperature and pressure across the mold, cooling to define cycle time, and assessment of weldlines. The PBT enters the mold from a gate at the base of the component and gives an even fill in approximately .5 second. Mold cooling was adapted to ensure that the core pin remains straight as the part hardens. A cap is ultrasonically welded at one end of the part after molding to hold an O-ring.
Ticona, Summit, NJ
Phone: (800) 833-4882
Web: www.ticona.com
 High-temp compound endures extreme conditions
High-temp compound endures extreme conditions
Aladdin Temprite's Insul-Plus base, constructed from a high-impact thermoplastic, is used primarily in hospitals and health care facilities to keep plates of food hot during distribution to patients and residents. The base is constructed of an injection molded top and a bottom shell that contains a wax-filled bladder. The wax melts during the preheating process, and as it solidifies, heat is given back to keep the food warm.
The bases are expected to last at least three years and are subject to constant heat pressure. "They are heated three times a day in a convection cabinet at 235F (113C) for up to 2 hours. It totals more than 6000 hours of heat exposure," says Burk Wyatt, manager of research and development at Aladdin Temprite. "In addition to heat, the bases are exposed to dishwasher detergents and rinsing agents in commercial dishwashers three times a day. They must also stand up to rough handling and stacking."
RTP has produced a 1400 Series polyethersulfone compound for the application that can withstand extreme temperature, chemical, and handling conditions. The material has an Izod impact strength at 1/8 inch of 11 ft-lb/in. It also has a heat deflection temperature of 410F at 264 psi.
Bill Durden, vp and gm of molder Durden Enterprises, describes the manufacturing process. "This material is processed at temperatures exceeding 700F. Once the shell is at room temperature, we sonic weld the perimeter as well as the center to prevent bulging during the heating process. The cycle time for each piece is about 50 seconds because of the large circumference of this part."
In contrast to traditional stainless steel bases, RTP says the thermoplastic Insul-Plus offers longer-lasting performance at a lower cost, and its lighter weight eases handling and reduces the load on delivery carts.
RTP Co., Winona, MN
Phone: (507) 454-6900; Fax: (507) 454-8130
Web: www.rtpcompany.com
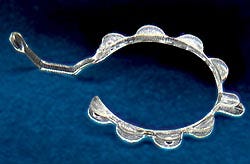 Pupil dilation tool simplifies surgery
Pupil dilation tool simplifies surgery
Some cataract surgery patients now have a better chance at a successful operation the first time thanks to an invention called the Perfect Pupil. Invented by John Milverton of Milvella Ltd. (Sydney, Australia), the tool, a nearly microscopic circular spring, is designed to expand the pupil in cases where the pupil opening is too small to effectively remove the cataract lens and debris and replace it with an implanted intraocular lens. Previously, followup procedures were required to correct or repair the resulting damage to the pupils.
Milvella's Geoff Neilson approached MediCon Inc., now Biomec Cardiovascular, to mold the part, complete the development, and manufacture the finished product. Working with S&W Plastics LLC (Eden Prairie, MN), MediCon developed the tool design, manufacturing processes, quality systems, and materials selection process. Premier Tool (Fridley, MN) built the mold, which Larry Lundeen, president of MediCon, says required microscopic detail and precision.
Selecting the right material proved to be a challenge, because it had to fill thin-wall sections and needed to give the part spring action. The latter property required a specific flex modulus and the ability to return the part to its original shape after being wrapped around a pin. Finally, the weight had to be similar to that of water so that it could float. Acrylic was the first material chosen based on its published physical properties and melt flow analysis results, but it did not carry the required spring condition in testing. MediCon then tried a polyurethane from Dow, and it produced a proper part.
When it came to shooting plastic, the molder felt that a custom injection unit was necessary to open the process window to allow for better parts and more consistent yield. Novel BioMedical (Plymouth, MN) built a unit based on Lundeen's design that can mold parts with wall sections as thin as .005 inch. Pressures were reduced by 45 percent, material temperatures by 15 percent, and volume of resin by 75 percent, which cut residence time, degradation, and contamination. Because the injection speed of the resin also increased, MediCon was able to reduce the number of gates from two to one (which is cut with a laser), thereby reducing knitlines. The Perfect Pupil is molded on an optimized tabletop micromolding machine from Novel.
Dow Plastics, Midland, MI
Phone: (800) 441-4369
Web: www.dow.com
 High-purity polymer provides wear resistance
High-purity polymer provides wear resistance
To ensure precise dosage and flow of dialyzing liquid in its hemodialyzers—used for treating patients whose kidneys are functioning at less than 5 percent of normal—Fresenius Medical Care Deutschland GmbH (Schweinfurt, Germany) chose a polyaryletherketone from Victrex. Intended for the gear wheels of dialyzer pumps, the polymer offers high purity in combination with reportedly good tribological properties and heat and chemical resistance.
The dialyzer extracts harmful metabolic substances and toxins from the patient's bloodstream in an artificial external process. The blood flows through filters in the dialyzer, where the lower-molecular-weight substances diffuse through membranes and are subsequently collected and removed by the dialyzing liquid in cross flow.
Dialysis is normally performed three times a week during sessions lasting 4 to 5 hours each. Because the machines are frequently cleaned and disinfected using a variety of chemicals, the gear material had to exhibit excellent chemical resistance, particularly against active chlorine, oxygen, and aldehyde disinfectants. In addition, chemicals such as citric acid are used for hot cleaning the equipment at temperatures of about 185F (85C).
In contrast to many amorphous plastics, the semicrystalline structure of the polyaryletherketone resin gives it stress crack resistance to a range of disinfectants. "The polymer can withstand more than 1000 cycles of cleaning and saturated steam sterilization at 273F (134C), without any significant loss of mechanical properties," reports Kevin Jennings, gm for Victrex North America.
Wear and abrasion resistance is important to prevent the dialyzing liquid from particle contamination. "The gear wheels must also provide a degree of dimensional accuracy that can only be achieved by machining the molded parts," says Jennings. "Extreme flatness and clean surfaces are among the indispensable requirements for this application."
Victrex USA Inc., West Chester, PA
Phone: (800) 842-8739; Fax: (610) 696-5702
Web: www.victrex.com
You May Also Like


