The Materials Analyst, Part 114:
The challenge of bonding plastic components in medical devices
Assembling parts made of amorphous resins means sorting out the conundrum between adhesion and ESC or chemical failure.
February 17, 2010
Assembling parts made of amorphous resins means sorting out the conundrum between adhesion and ESC or 
chemical failure.
Medical devices use a wide variety of polymeric materials. Some of these materials are relatively rigid at room temperature, such as PC and acrylic, while others, like silicone and plasticized PVC, are elastomeric. They are soft and pliable at ambient conditions and are used in the form of tubing and other flexible components.
|
In many cases the polymers used in medical devices are amorphous for a number of reasons. First, clarity is a desirable characteristic for many devices so that fluid paths can be visually verified. Second, amorphous polymers tend to have better impact resistance and are less likely to undergo catastrophic fracture in the application environment. In addition, amorphous polymers exhibit low and predictable levels of shrinkage during the molding process. This makes it easier to mold parts to close tolerances. Finally, assembly techniques have often favored some form of solvent or adhesive bonding and amorphous resins accommodate this type of treatment more readily than the more chemically resistant semicrystalline resins.
This last consideration, however, is a double-edged sword. The very characteristics that make amorphous polymers amenable to solvent and adhesive bonding also make them susceptible to the leading cause of plastic product field failures, environmental stress cracking. And in more extreme cases they may also exhibit chemical attack. It is worth taking a moment to distinguish between these two failure modes, because they are often thought of as being equivalent. However, they are very different mechanisms and must be solved using different strategies.
Know your causes
Chemical attack is relatively straightforward. It represents an inherent chemical incompatibility that usually takes the form of a reaction between a polymeric material and a chemical. It may result in solvation of the polymer, but often it also appears as a loss in ductility with an associated formation of cracking and a change in the surface appearance of the material. One of the most dramatic examples of chemical attack is the interaction between foamed polystyrene and acetone. Poured into a Styrofoam cup, acetone will eat through the bottom of the cup in a very short period of time. This will happen every time contact between the plastic and the chemical occurs.
Environmental stress cracking (ESC) is a very different proposition. In ESC, stress and a chemical agent work together to produce failure that generally takes much longer to manifest. Neither influence by itself is sufficient to produce the failure. Removing the chemical or even reducing its concentration will provide a remedy even if the stress is still present. Conversely, reducing or removing the stress will solve ESC problems even if the chemical remains present.
|
Fundamentally, ESC is the mechanically driven process of creep that is accelerated by the presence of the chemical. Stresses can come from the application environment, they can be caused by poor design elements such as sharp corners or sudden changes in wall thickness, or they can come from processing conditions such as quench cooling or overpacking of the mold cavity. And because, by definition, stress is measured as a force per unit area, the actual stress in a part is a function of part geometry.
Consequently, ESC can be solved by a number of routes that include changes to the part design and the process as well as changes in the material selected while chemical attack requires either removal of the chemical or a change in the polymer used to make the part.
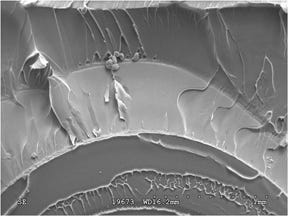
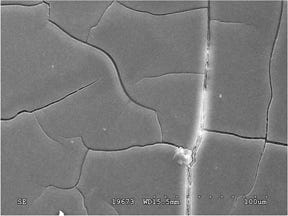
Because of the fundamental differences between these two mechanisms, it is critical that a root cause analysis distinguish between them if a product failure is to be solved cost effectively. Under magnification, cracks caused by chemical attack look different from those caused by ESC, as shown in Figures 1 and 2. Because ESC has a stress component, there is a clear directionality to the fracture extension process.
Another hallmark of ESC is the tendency for the fracture to initiate at multiple, closely spaced locations. Chemical attack produces signatures such as mud cracking. Because stress is not a required element, the fracture surface will often lack an indication of a specific origin or a direction of crack growth. In both cases these mechanisms can make an inherently ductile material appear to be quite brittle. This is indicated by the relatively smooth features of the fracture, an indication that the stresses were relatively low and yet still resulted in failure. It may be surprising to know that the material in both of these parts is polycarbonate (PC), the same material that can withstand the impact of a bullet.
Both of these parts failed at much lower stress levels than those associated with a high-speed projectile collision, and they both failed as the result of an assembly process that uses bonding agents to join components in a medical device. The conundrum in the bonding process is that the very chemicals needed to produce a good bond also have the capability of inducing these types of failures. This is because the chemicals, whether they act to form a solvent bond or whether they employ an adhesive that cures, can chemically attack or stress crack the polymers used in the device. Stress cracking is much more common, and the mechanism has been well quantified for materials like PC precisely because the problem is so pervasive.
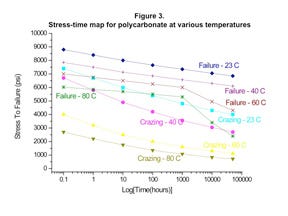
Figure 3 provides a plot of the stress required to produce failure in PC as a function of time, temperature, and the presence or absence of a chemical capable of producing the initial stages of stress cracking known as crazing. It is not a big surprise to see that at room temperature (23°C), the stress required to produce almost immediate failure in PC is approximately 9000 psi, the typical yield strength for the material. The introduction of a chemical capable of producing stress cracking reduces even this short-term value to 7400 psi.
When time is factored into these considerations, the effects of the chemical on the stress tolerance of the material become more pronounced. At the 10,000-hour point, the stress required to produce failure in PC that is not exposed to a stress crack agent has declined to 7250 psi, or by about 20%. This is essentially a measure of the creep resistance of the polymer. However, the stress tolerance for the exposed material has declined to 4300 psi, or more than 40% from the short-term performance value.
Another way of looking at these data is to equate the performance of unexposed PC and exposed PC at the same stress level. This produces the observation that the same stress level that produces failure in an unexposed PC in 10,000 hours produces almost immediate failure in the polymer when exposed to a stress crack agent.
When increased temperature is brought into the picture, the problems associated with exposure to stress crack agents become even more acute. Many medical devices undergo sterilization. While high-energy techniques such as gamma and E-beam irradiation are becoming increasingly popular, some products are still sterilized by ethylene oxide. This technique involves exposure to temperatures as high as 60°C for 18-36 hours.
While this is a relatively short time period, a review of the data in Figure 3 shows that it is not trivial. Simply raising the temperature of the environment has a significant effect, reducing the short-term stress tolerance from 9000 to 7000 psi for the unexposed polymer and from 7400 to 4000 psi for a material in contact with a stress crack agent. After just 20 hours, the unexposed material maintains more than 90% of this initial value at 6400 psi while the exposed material will now fail if exposed to a stress greater than 2300 psi. The implications for a designer using finite element analysis (FEA) to identify safe stress levels are clear.
Old enemies: time and temperature
Recently, the effect of chemicals used in solvent bonding was examined in a different way using dynamic mechanical analysis (DMA). The results were striking and offer another way of looking at how the performance of a material like PC is influenced by common assembly processes used in the medical device industry. DMA measures the viscoelastic properties of a material as a function of various parameters. The most common of these is temperature. A temperature scan of a material will produce a plot of elastic properties, viscous properties, and the ratio between them (referred to as tan delta) as a function of temperature.
The temperature scan has value in and of itself. However, if we understand that in viscoelastic materials, the effects of time and temperature on relaxation processes are equivalent, then we can also use the temperature-dependent behavior to make inferences about behavior at various temperatures over extended periods of time. A material that exhibits little change in elastic modulus with temperature will tend to exhibit good creep resistance in that temperature region. In temperature regions where large changes in elastic modulus are approaching or are already occurring, the tendency for large deformations under load over time will increase and with it the likelihood of failure.
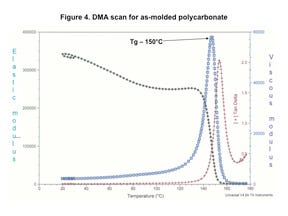
Figure 4 shows a DMA scan for an as-molded PC. The results are typical for PC. The elastic modulus at room temperature is 340 kpsi, which is in excellent agreement with data sheet values derived from classical tensile and flexural tests. The elastic modulus declines by approximately 20% between room temperature and 135°C before declining rapidly between 135°C and 155°C. This narrow temperature region is known as the glass transition.
Note that as the temperature increases and the elastic modulus approaches the glass transition, the viscous modulus rises steadily, rising gradually at first and then much more rapidly in the glass transition region. For convenience, we identify the glass transition temperature (Tg) as the peak of the viscous modulus curve, although an examination of the graph clearly shows that this is a process that takes place over a temperature range rather than a true phase change like ice melting. Part of the reason for the observed reduction in stress tolerance with time that is shown in Figure 3 is related to the proximity of the application temperature to the Tg.
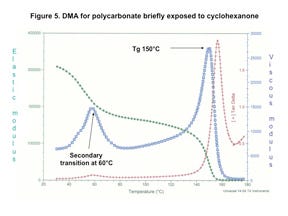
But Figure 5 shows drastically different behavior once the PC has been exposed to cyclohexanone, one of the chemicals commonly used to produce bonds between plastic components in medical devices. The modulus at room temperature is already lower due to this exposure, measuring 315 kpsi. However, of much greater importance is the presence of a preliminary relaxation that occurs between 40°C and 80°C with a peak near 60°C. The elastic modulus reduction associated with this transition represents a 40% decline and is associated with a greater degree of molecular mobility within the polymer matrix as a result of contact with the cyclohexanone. This provides insight into the structural causes for the loss in mechanical performance shown in Figure 3.
This situation highlights a considerable dilemma for the medical device industry. The ability to solvent bond amorphous polymers is an advantage that has shaped the assembly processes in the industry. However, this ability brings with it an inherent weakening of the polymers that can lead to failure of key components. UV-curable adhesives offer an alternative mechanism; however, research has shown that in the uncured state these adhesives also contain aggressive constituents that can promote stress cracking. In addition, the use of adhesives introduces another substance with its own property profile. This can influence the performance of the entire device if the properties of the adhesive are not known or properly accounted for.
With the increasing importance of medical devices to an aging population and the increased use of plastic materials in these devices, these challenges will not lessen. However, they may provide the impetus for new approaches to medical device assembly that include performing some of these operations in the mold. Justifying this will, as usual, be a matter of balancing cost and performance. However, an improved understanding of the effects of secondary processes such as assembly and sterilization will be an important component of the decision-making process. —[email protected]
About the Author(s)
You May Also Like