Contact Lens Technology Enables Monitoring of Medical Conditions
The device embedded with stretchable biosensors to help diagnose and monitor medical conditions may soon be ready for clinical trials.
March 11, 2021
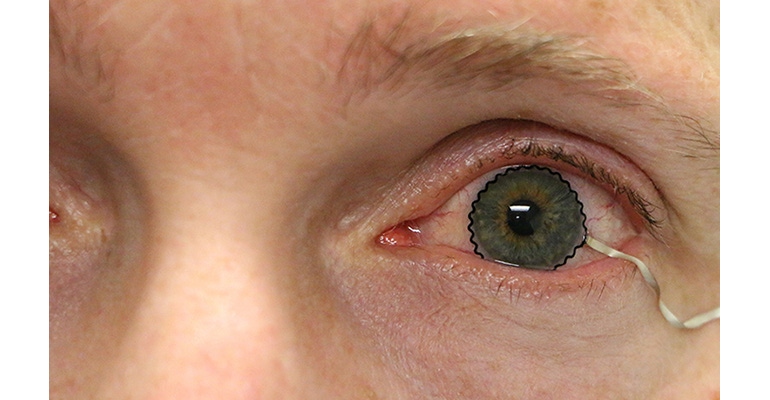
New contact lens technology integrated with stretchable biosensors to help diagnose and monitor medical conditions may soon be ready for clinical trials, reports Purdue University on its website. The device represents an alternative to current techniques that typically involve topical anesthetics or a speculum, which are uncomfortable and often painful for the patient.
Researchers from Purdue University worked with biomedical, mechanical, and chemical engineers, along with clinicians, to develop the novel technology. The team turned commercial soft contact lenses into bioinstrumentation tools for unobtrusive monitoring of clinically important information associated with underlying ocular health conditions, writes Chris Adams in the article.
The breakthrough was enabled by the seamless integration of ultrathin, stretchable biosensors with commercial soft contact lenses via wet adhesive bonding. Sensors or other electronics previously couldn’t be used for commercial soft contact lenses because the fabrication technology required a rigid, planar surface incompatible with the soft, curved shape of a contact lens. The biosensors used by Purdue researchers record electrophysiological retinal activity from the corneal surface of human eyes, without the need of topical anesthesia that has been required in current clinical settings for pain management and safety.
The sensor technology is described by the researchers in a paper published this week in Nature Communications. Here is an excerpt:
“The corneal sensor is configured into a thin, narrow serpentine trace . . . and positioned on the inner surface of a commercial disposable [soft contact lens] (SCL) facing the corneal surface. A conductive biocompatible polymer, poly(3,4-ethylenedioxythiophene) (PEDOT) doped with tosylate is electrochemically printed around the entire outer surface of the corneal sensor in order to provide a thin encapsulation layer and promote anchoring to the SCL. The corneal sensor is monolithically linked to an elastomeric connection wire . . . that comprises custom-formulated elastomers, including silver flake-filled polystyrene-b-poly(ethylene-co-butylene)-b-polystyrene (AgSEBS) and fumed silica nanoparticle-filled polydimethylsiloxane (PDMS). Here, the connection wire penetrates through the SCL for seamless integration.” The full paper in Nature Communications can be read here.
“This technology will be greatly beneficial to the painless diagnosis or early detection of many ocular diseases including glaucoma,” said Chi Hwan Lee, Purdue’s Leslie A. Geddes assistant professor of biomedical engineering and assistant professor of mechanical engineering, who is leading the development team. It will allow “doctors and scientists to better understand spontaneous retinal activity with significantly improved accuracy, reliability, and user comfort,” added Pete Kollbaum, director of the Borish Center for Ophthalmic Research and an associate professor of optometry at Indiana University, who is leading clinical trials.
About the Author(s)
You May Also Like