From Design to FOT Molded Medical Device in Three Weeks
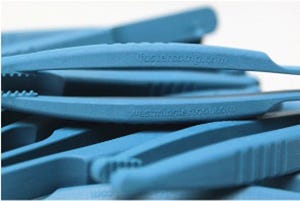
Medical-grade forceps are reverse engineered and molded from a bio-based polymer in record time thanks to 3D-printed inserts.
April 11, 2022

For several years now, Westminster Tool has explored the use of metal additive manufacturing in moldmaking applications in partnership with 3D-printing technology company Mantle Inc. On their most recent project — the production of medical-grade forceps using metal 3D-printed cavities — the companies were joined by Foster Corp., which provided a new glass-filled, bio-based polymer designed for medical devices.
|
The forceps are molded from a new glass-filled, bio-based polymer. |
Using Foster’s latest polymer from Arkema, Rilsan FKZM 65 O TD MED, Westminster Tool worked with Mantle to design and produce metal 3D-printed cavities for research and development. “The idea is that we could create a production-environment prototype tool quickly, and Foster would get real-world medical device examples for potential customers,” explained Westminster Tool Manufacturing Engineer Eddie Graff. Graff worked with engineers at both Mantle and Foster to see the project through to completion. “We’ve worked with glass-filled medical resins before, so we already had that experience, but this material was really unique.”
Aluminum prototype tool ruled out
The material’s high-density glass filling made it very abrasive and unsuitable for an aluminum prototype tool, explained Westminster and Mantle in the case study. “Aluminum tools do not have the rigidity or lifespan of steel tools and typically do not have conformal cooling,” noted the paper. “When an aluminum tool is used with a highly glass-filled abrasive material, there is risk of rounding the edges of the tunnel gate, which can leave a messy gate vestige, or extra plastic in the gate area. This visual defect can continue to worsen over tens of thousands of shots. Instead, Mantle’s H13-equivalent printed cavities became a popular alternative, offering more longevity for testing the molding process.”
In addition to allowing for the use of a subgate to mold thousands of parts without risking gate erosion, the 3D-printed cavities enabled Westminster Tool to put in cooling lines. Conventional methods would have required building the cavity as two separate pieces and welding them together to achieve conformal cooling, according to Westminster Tool. Without conformal cooling, experimentation of molding parameters with an aluminum tool would not have been possible without dramatically impacting cycle time.
Bio-based material developed specifically for surgical devices
|
The 3D-printed cavities enabled Westminster Tool to put in cooling lines. |
As with many new materials, all that was provided for the initial design kick-off were sample parts and technical data such as material characteristics and processing conditions. According to commercial information from Arkema, the material was developed specifically with surgical instruments in mind. Not only does the material offer high performance but emphasizes sustainability, being engineered from castor oil. “We were really eager to work with a renewable resin like this,” said Graff.
Westminster Tool was able to review the sample parts, technical info, and reverse engineer medical forceps designed from an additive manufacturing perspective. “Designing for additive is a completely different mindset,” explained Graff. “It’s challenging because you’re considering features that would never have been possible with machining. Not even EDM or 5-axis milling. From the very start, we approached this like any major customer, with an in-depth DFM process and a full-scale DCII process development to determine ideal molding parameters for quality parts,” added Graf.
By using Mantle to print the cavities, and with minor post-processing such as grinding, milling, and EDM, Westminster Tool was able to perform first-out-of-tool molding in just three weeks. The printing of the cavities alone — both sides being printed simultaneously — only took roughly 86 hours. “That’s three weeks to get quality medical parts from design to a prototype tool. It’s incredible,” Graff said.
You May Also Like