Swiss medical device company develops world's first bioabsorable heart valve
Conventional heart-valve replacement procedures have a number of challenges beyond the operation itself: The long-term presence of a foreign material in the body can cause inflammation and revision surgery is typically needed, driving up healthcare costs and increasing risk for the patient. Xeltis (Zurich, Switzerland) has developed a technology that uses bioabsorbable polymers structured as a porous matrix that promote the body's natural healing process and are resorbed once the new functioning tissue has been formed.
December 1, 2015
Conventional heart-valve replacement procedures have a number of challenges beyond the operation itself: The long-term presence of a foreign material in the body can cause inflammation and revision surgery is typically needed, driving up healthcare costs and increasing risk for the patient. Xeltis (Zurich, Switzerland) has developed a technology that uses bioabsorbable polymers structured as a porous matrix that promote the body's natural healing process and are resorbed once the new functioning tissue has been formed. The company has successfully completed its second successful feasibility trial and is a darling of the investment community. It is aiming for CE mark approval for a pulmonary valve in 2018, which would allow Xeltis to market its product throughout the European Union and other countries that recognize the marking.
|
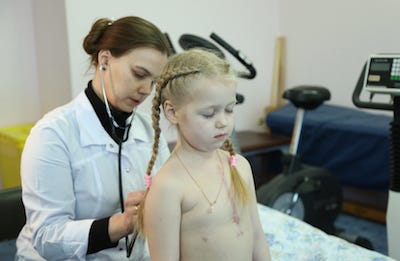
Dominika Zhurkin, a patient in the first Xeltis feasibility study, undergoes |
The company's proprietary Endogenous Tissue Restoration (ETR) technology is based on Professor Jean-Marie Lehn's Nobel Prize–winning research on supramolecular chemistry. In essence, the bioabsorbable materials are designed to work as actual heart valves, vessels or tissue once implanted, and to begin the bioabsorption process once the body has produced enough tissue to take over the mechanical function of the valve, writes the company on its website.
In the company's most recent feasibility study, a Xeltis cardio-vascular patch was implanted in young children who were born with a single functioning heart ventricle. Results showed that all of the patients presented no post-operative complications or functional impairment requiring intervention one year after surgery, according to the company. Although the potential patient population for that device is limited, Xeltis CEO Laurent Grandidier stresses its value as a stepping stone in the development of other bioabsorbable cardio-vascular devices. "The technology we are developing is not a one-product technology—it's a platform technology that can be applied to a multitude of cardio-vascular treatments," he told European CEO recently. And that has investors excited.
Private equity company LSP (Amsterdam) co-led a €27 million Series B fund in 2014, one of the largest investments to date by the premier European healthcare investor. What LSP and like-minded financiers see in the company is its alignment with the new healthcare business paradigm: Improving outcomes in a cost-effective manner.
Xeltis is now developing its first commercial product: A pulmonary valve to treat children born with severe heart malformations. "However, the platform technology we are developing can be used for other applications," Grandidier told European CEO. "So we also have R&D projects for adult applications in the heart-valve space."
About the Author(s)
You May Also Like