A safety device that protects healthcare workers from needlestick injuries received the DeviceMed award for most innovative medical device at Compamed, the medical manufacturing event co-located with Medica, the world's largest medical tradeshow, in Düsseldorf, Germany, last month. Jointly developed by Cambridge Design Partnership (Cambridge, UK) and Raumedic AG (Helmbrechts, Germany), the RauSafe device activates a telescopic sleeve following injection to cover the needle.
December 16, 2014
A safety device that protects healthcare workers from needlestick injuries received the DeviceMed award for most innovative medical device at Compamed, the medical manufacturing event co-located with Medica, the world's largest medical tradeshow, in Düsseldorf, Germany, last month. Jointly developed by Cambridge Design Partnership (Cambridge, UK) and Raumedic AG (Helmbrechts, Germany), the RauSafe device activates a telescopic sleeve following injection to cover the needle.
Each year 385,000 needlestick injuries and other sharps-related injuries are sustained by hospital-based healthcare workers in the United States, according to Cambridge Design Partnership. Both the USA Needlestick Act (2000) and EU Needle Safety Directive (2010) came into force to help prevent sharps injuries in hospitals. As many existing prefilled glass syringes on the market did not meet these needle safety regulations, pharmaceutical companies faced lengthy and costly processes to redesign and revalidate their drugs in new needle-safe primary pack delivery devices.
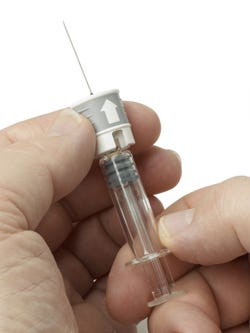 Recognizing the need for a needle safety device that could be retro-fitted to existing primary packs and avoid a lengthy and expensive revalidation process, Raumedic and Cambridge Design Partnership put their heads together and developed a solution in just five months.
Recognizing the need for a needle safety device that could be retro-fitted to existing primary packs and avoid a lengthy and expensive revalidation process, Raumedic and Cambridge Design Partnership put their heads together and developed a solution in just five months.
Cambridge Design Partnership carried out initial technology and IP landscaping to identify areas of opportunity, then concept invention and mechanical CAD development to create a range of concepts. In addition, using its core toolkit of processes for design for manufacture and assembly, tolerancing and stress analysis, and working in partnership with Raumedic's injection molding experts to prototype the device, the team arrived at a robust design suitable for high volume manufacture at competitive cost.
The RauSafe can be customised to accommodate existing staked-needle prefilled glass syringes with slight modifications to device parts. This means it can be added to existing manufacturing lines, avoiding a lengthy revalidation process.
RauSafe is currently available for license.
About the Author(s)
You May Also Like


