Sintering: A critical step
October 24, 2002
Editor’s note: A recognized expert in powder injection molding (PIM), Randall German is Brush Chair Professor in Materials at Penn State University.
|
|
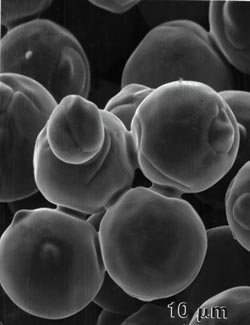
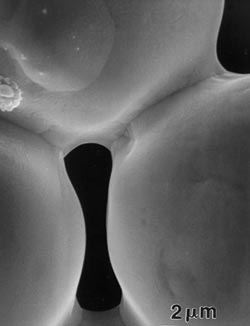
Figure 1. Two scanning electron micrographs of sinter bonds formed between 32-µm nickel particles originally in point contact, heated to 1000C in a vacuum for 30 minutes. |
PIM takes advantage of the fluid-like behavior of a heated powder-polymer feedstock to form complex shapes. It can be performed with wax-like polymers and any sinterable alloy, ceramic, or inorganic powder. After moldfilling, the polymer is solidified to hold the mold shape, but the powder-polymer mixture has little strength beyond that required for ejection. Subsequently, the polymer (binder) is removed using heat, solvents, or catalysts and the powder is sintered to nearly full density. Sintering is a heat treatment that is applied to the powders to strengthen and densify the particles.
The sintering process has been known for thousands of years, and was first used to create stronger bricks and gold-platinum jewelry. Modern uses for sintering trace their origins to the 1910 to 1930 time period when tungsten lamp filaments, ceramic spark plug bodies, tungsten carbide cutting tools, porous bronze bearings, alumina-silica electrical insulators, and tungsten-copper electrical contacts were developed. By the 1940s, sintering was used to fabricate uranium dioxide nuclear fuel elements, porous nickel filters, steel artillery projectile bands, and eventually steel automotive components.
Today, sintering is employed in a diverse range of products that includes dental implants, automotive valve seats, rocket nozzles, aircraft wing weights, ultrasonic transducers, ceramic turbochargers, and golf clubs. Many of the modern applications are justified by factors such as manufacturing economy, improved properties, and novel compositions. This is where PIM comes into play, since it provides an economical means to shape a powder in a mass production environment. Present-day focus is on high-performance materials—steels, titanium, special ceramics, electronic alloys, cemented carbides, refractory metals, and other advanced engineering materials.
Vanishing Pores
In the microscope we see evidence of sintering by the growth of bonds or necks between particles. These bonds grow spontaneously by atomic motion induced by the high sintering temperature. Figure 1 (right) is a scanning electron micrograph of the sinter bond formed between nickel particles heated for 30 minutes to 1000C. This bond grows due to random high-temperature, atomic-level events that act to eliminate the pore surface area between the particles.
Sinter bonding of PIM particles typically occurs at temperatures in excess of about one-half of the absolute melting temperature. For example, first sintering occurs for a steel powder at about 600C, but full density and good properties require 1250C. Since engineering materials melt over a wide range of temperatures, there is a corresponding wide range of sintering temperatures; what works for sintering aluminum (620C sintering and 660C melting) is too cool for tungsten, which requires sintering near 2200C because of its 3410C melting temperature.
Sintering occurs because the powders have excess surface energy associated with the particle surface. Just as two soap bubbles spontaneously bond, two particles join if heated to a high temperature. These sinter bonds grow by atomic-level events, one atom at a time. Fortunately, as temperature increases the atoms become very mobile and at the typical sintering temperature each atom jumps to a new position six times every second.
|
Figure 2. An iron-nickel alloy magnetic valve body before (right) and after (left) sintering, showing the dimensional change associated with elimination of pores during sintering. In this case the shrinkage is about 15 percent of the initial dimension. |
Through random atomic motion the pores are progressively filled, one atom at a time. For typical PIM parts the pore elimination requires about 1 hour. Even so, humans (and customers) are impatient, so we invented faster sintering techniques using liquid phases. Atomic motion in a liquid is about 100 times faster than in a solid, so productivity can be improved by creating a molten brazed layer between the solid particles. This technique is known as liquid phase sintering and is used for about 70 percent of all sintered products.
Shrinkage and Other Factors
Sintering is an irreversible process. Once a powder is heated it cannot revert back to powder. There is significant pore loss between the particles, easily seen by shrinkage in external dimensions. For many PIM components this is about 15 percent dimensional change (Figure 2).
Most engineering properties undergo dramatic improvements during sintering due to pore loss, including major changes in strength, ductility, hardness, electrical conductivity, thermal diffusivity, magnetic permeability, and corrosion resistance. These property gains are the primary justification for sintering. One option in PIM is to mix different powders together in the feedstock to form unique, multiple-phase sintered structures with novel property combinations.
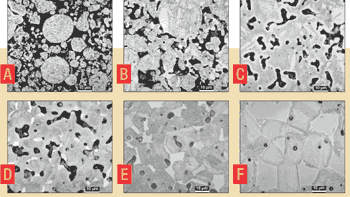
Figure 3. Optical microscope images taken from a stainless steel air compressor component formed by PIM. This image sequence was generated by quenching the material from various temperatures during the sintering cycle. The pores are black and diminish in size and content during heating to progressively higher temperatures: a) 1000C, b) 1100C, c) 1200C, d) 1260C, e) 1300C, and f) 1365C (the final sintering temperature). | |
A microstructure view of sintering is given in the set of pictures shown in Figure 3. These were taken from an injection molded stainless steel air compressor component. The part was quenched from various temperatures during heating to the sintering temperature to capture the state of bonding between the particles. Each quenched piece was embedded in epoxy, sectioned and polished, and photographed in a microscope. Note that the content of black pores diminishes as temperature increases, to the point where essentially all of the pores are eliminated by the end of the sintering treatment. This stainless alloy has two different phases (or crystal structures), leading to an interesting tapestry in the sintered microstructure. Curiously, a powder can be oversintered, producing a structure in which the properties decline with heating for longer times or at higher temperatures.
Realize that small powders have a high surface area, so they naturally sinter faster than large powders. Indeed, all particles have an inherent driving force termed the sintering stress, which can be estimated by dividing the surface energy by the particle size. For most PIM powders, this gives a stress on the order of 1 MPa. However, this is only half of the story, since sintering requires mass flow to respond to this stress. Hence most powders will not sinter at room temperature.
Broad Applications
Today, sintering is critical to several industries and contributes more than $23 billion to the U.S. economy each year. A few large actors are in the production of automotive steel engine components, tungsten carbide indexable inserts for metalcutting, ceramics insulators for electrical systems, and electronic capacitors. Almost all common engineering materials can be sintered. Consequently, sintered products can be tailored for a wide range of engineering properties and applications.
In this light we see that PIM is an intersection of two complementary technologies—plastics molding for shaping and sintering for strengthening.
Contact information |
You May Also Like