Polyurethane foam enables medtech manufacturers to meet broad range of requirements
Plastic materials used in medical applications must meet rigorous performance specifications, but increasingly they also must satisfy ergonomic and aesthetic requirements. And then there are cost considerations. Bayer MaterialScience (Leverkusen, Germany) has published a press release describing how its polyurethane integral skin foam materials meet functionality, design, strength, and fire protection requirements in a specific medical application.
August 22, 2014
Plastic materials used in medical applications must meet rigorous performance specifications, but increasingly they also must satisfy ergonomic and aesthetic requirements. And then there are cost considerations. Bayer MaterialScience (Leverkusen, Germany) has published a press release describing how its polyurethane integral skin foam materials meet functionality, design, strength, and fire protection requirements in a specific medical application.
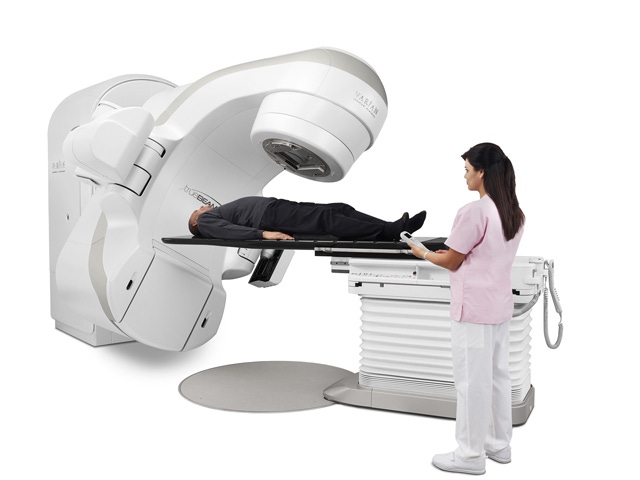
 Emaform AG (Gontenschwil, Switzerland), a supplier of molded parts and housings in rigid integral skin polyurethane foam and microcellular polyurethane, uses Baydur 66 FR and 110 FR from Bayer MaterialScience to fabricate parts for the TrueBeam system from Varian Medical Systems (Cham Switzerland). Headquartered in Palo Alto, CA, Varian is a leading manufacturer of medical technology for treating cancer and other medical conditions with radiotherapy, brachytherapy, and radiosurgery.
Emaform AG (Gontenschwil, Switzerland), a supplier of molded parts and housings in rigid integral skin polyurethane foam and microcellular polyurethane, uses Baydur 66 FR and 110 FR from Bayer MaterialScience to fabricate parts for the TrueBeam system from Varian Medical Systems (Cham Switzerland). Headquartered in Palo Alto, CA, Varian is a leading manufacturer of medical technology for treating cancer and other medical conditions with radiotherapy, brachytherapy, and radiosurgery.
Today's medical technology products are often complex devices, notes Bayer MaterialScience. This also applies to housings that protect internal components from environmental conditions and contamination. Emaform employs an integral construction method, with bushings and threads included in the molding process. Brackets, sheet metal, and Plexiglas parts are bonded to the molding, and various parts are then screwed or bolted on to the assembly.
Vertically integrated manufacturing allows Emaform to supply complete assemblies, eliminating some final assembly operations for Varian. The partnership also allows Varian to offer hospitals and other end users efficient spare parts management.
Decorative and ergonomic considerations are also facilitated by the use of Baydur materials, says Bayer MaterialScience, as they offer design freedom while meeting manufacturer requirements for high-quality moldings.
Baydur 66 FR produces sandwich structures with stiffness and low weight properties, thanks to the hard surface and microporous structure inside the components. Baydur 110 FR is suited for thin-walled parts with a high-quality, stable surface.
The option of using low-cost aluminum molds offers a cost saving over thermoplastic materials, as polyurethane moldings are manufactured at much lower pressures.
About the Author(s)
You May Also Like


