The right plastic can prevent the wrong medical connection
Luer connectors have been a mainstay in small-bore--defined as inner diameters smaller than 8.5 mm--medical applications for decades. The universal design has facilitated connecting medical devices that deliver fluids, gases, medication and nutrients to patients, but the convenience also has a drawback: Misconnections are easily made, and that can have tragic consequences. The new ISO 80369 standard has been developed to prevent the interconnectability of unrelated delivery systems.
June 8, 2015
Luer connectors have been a mainstay in small-bore--defined as inner diameters smaller than 8.5 mm--medical applications for decades. The universal design has facilitated connecting medical devices that deliver fluids, gases, medication and nutrients to patients, but the convenience also has a drawback: Misconnections are easily made, and that can have tragic consequences. The new ISO 80369 standard has been developed to prevent the interconnectability of unrelated delivery systems. This is achieved by standardizing connectors for specific devices, as PlasticsToday reported recently, but material selection also plays a role. Eastman Chemical Co. (Kingsport, TN) explains.
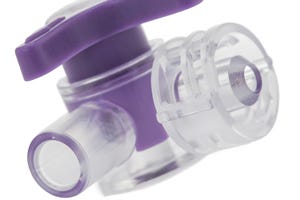 Prior to ISO 80369, flexible materials such as PVC, TPEs and TPUs were typically used to mold connectors, and healthcare personnel—rushed and, often, dealing with emergencies—were known to force together connectors by stretching the material. To prevent this, "the standard prescribes engineering resins with a modulus of 700 MPa or greater that won't deform as easily as the flexible materials used in the past," says Steven Givens, PhD, Senior Polymer Application Scientist, Eastman Chemical. The company's co-polyester Tritan meets this requirement, notes Givens. Moreover, the material is chemical resistant and is compatible with solvent bonding, properties that also benefit medical connector applications.
Prior to ISO 80369, flexible materials such as PVC, TPEs and TPUs were typically used to mold connectors, and healthcare personnel—rushed and, often, dealing with emergencies—were known to force together connectors by stretching the material. To prevent this, "the standard prescribes engineering resins with a modulus of 700 MPa or greater that won't deform as easily as the flexible materials used in the past," says Steven Givens, PhD, Senior Polymer Application Scientist, Eastman Chemical. The company's co-polyester Tritan meets this requirement, notes Givens. Moreover, the material is chemical resistant and is compatible with solvent bonding, properties that also benefit medical connector applications.
"Chemical resistance is important," says Cynthia Lewis, Market Insight and Strategy Manager at Eastman, "because hospitals increasingly are using more aggressive disinfectants and cleaning materials to prevent hospital-acquired infections. Other materials used in small-bore connectors may crack when exposed to these harsher disinfectants," says Lewis.
The bond between the connector and PVC tubing that conveys fluids will fail under stress, adds Givens. That won't happen with Tritan. "Under stress, the bond between the connector hub and tubing won't fail. The tubing itself will snap, but the bond won't fail," says Givens.
Additional benefits of Tritan co-polyester in connector as well as other medical applications include its stability following sterilization—the process won't cause color shifts or brittleness, says Givens—and the absence of BPA and BPS, phthalates, halogens and heavy metals.
The material's profile and compatibility with the new standard has led German injection molder and moldmaker A. Hopf (Zirndorf) to use Tritan to produce enteral connectors (pictured) that will satisfy the forthcoming requirements. Eastman declined to name any other applications that may be in the pipeline.
Getting industry to go with the flow
ISO 80369 was developed by an international group of manufacturers, clinicians and regulators in collaboration with the International Organization for Standardization (ISO and the Association for the Advancement of Medical Instrumentation.
Currently, part 1 of ISO 80369 has been released, laying out the general requirements for small-bore connectors used in medical faciities to administer liquids and gases. Part 3, which defines the new enteral connector, has been published provisionally. Ultimately, the ISO document's various parts will set requirements for respiratory gas, enteral, urethra and urinary, blood pressure, neuraxial, and intravascular or hypodermic applications.
Lewis lauds ISO for taking a pragmatic approach by using IV tubing, the largest category, as a foundation. She also applauds the Global Enteral Device Supplier Association for executing an exemplary industry awareness campaign about the changes. Despite best efforts, however, some confusion does remain involving flow direction.
Traditionally, flow has been from a male to female connector. "Gravity pulls the fluid from a bag downward through a male connector into a female connector, which is normally attached to a patient," explains Givens. That is unchanged for IV connectors, but the ISO standard switches the direction for enteral feeding systems. "Going forward, in enteral feeding applications, the direction of fluid will be from a female connector to a male connector, making it impossible to interconnect by mistake an enteral feeding system to an IV system," says Givens.
This will require an adjustment on the part of medical personnel, but, as Lewis points out, a change in habits is a small price to pay for the end goal. "It's wonderful that industry is stepping up and supporting this effort to improve patient safety, which is what the standard was designed to do," says Lewis.
About the Author(s)
You May Also Like




