The Materials Analyst, Part 115: The hidden trade-offs between processing and performance
If the material is easier to run, there’s a good chance you’re giving up a valuable property advantage.While it may seem like a perverse principle, it is generally a true statement that in the world of injection molding, as processing becomes easier, the performance that can be expected from the material declines.
March 17, 2010
If the material is easier to run, there’s a good chance you’re giving up a valuable property advantage.
While it may seem like a perverse principle, it is generally a true statement that in the world of injection molding, as processing becomes easier, the performance that can be expected from the material declines.
One of the simplest illustrations of this is the trade-off between ease of mold filling and the properties of the final molded part. Typically, melt viscosity is a function of the molecular weight of the polymer. Melt viscosity increases with molecular weight and consequently the pressure required to fill any particular geometry increases with resins that have a higher molecular weight.
|
The feasibility of making any particular part is dependent on a complex interaction between the raw material, part geometry, gate location, processing conditions, and the capability of the molding machine to generate the necessary pressure. However, all things being equal, as molecular weight increases, the task of molding the part becomes more challenging. For all products there is a practical maximum molecular weight, beyond which the manufacturing process becomes untenable due to difficulties with filling and packing the mold. The payoffs for managing the higher-molecular-weight grades of a given polymer include improved toughness and enhanced resistance to long-term influences such as creep, fatigue, and environmental stress cracking.
A less well-known trade-off is one associated with the process conditions required to achieve a satisfactory degree of crystallinity. All semicrystalline resins require sufficient time to allow the polymer chains to organize so that a degree of crystallinity is achieved that is sufficient to allow for optimal part performance. Crystals can only form in the temperature window below the melting point and above the glass transition temperature (Tg). Polymers like polyethylene and polypropylene have glass transition temperatures below room temperature. This is indicative of a high degree of the type of mobility required to obtain a significant level of crystallinity, even at relatively low mold temperatures.
However, many semicrystalline polymers, such as nylons and polyesters, have higher Tgs. These materials require higher mold temperatures in order to attain the needed degree of crystallinity. A quick reading of the processing guidelines published by the suppliers of such materials shows that the primary processing condition that is used to achieve this optimal structure is mold temperature. Higher mold temperatures slow the rate of cooling and allow time for the polymer chains to organize into crystals. For materials like nylon 6 and PBT polyester, these mold temperatures will typically range from 70°C-100°C. Achieving these mold temperatures simply involves using water-circulating units that are set to a higher temperature than would typically be used for polypropylene (PP) or polyethylene (PE).
The mold must get hotter
However, there is another level of performance achievable with semicrystalline resins that have even higher Tgs. Materials like PPS, PEEK, and partially aromatic nylons have Tgs above the boiling point of water. To achieve the necessary time required to achieve the optimal level of crystallinity in these materials, the mold temperatures must be set in a range of 125°C-200°C, depending on the part geometry and the polymer being molded. These temperatures cannot be readily attained using water-circulating units because they exceed the boiling point of water; alternative methods such as oil heat, electric cartridges, or steam are required. This presents a significant technological barrier for many processors, and there is a discrete dividing line that separates processors who are willing to tackle these higher-tech approaches to mold temperature control from those who are not.
Part of this barrier involves cost. Oil heating units, for example, are approximately four times the cost of water-circulating units. In addition, the oil that is placed into these units must be purchased; it isn’t piped in “free” from the local municipality, and over time it undergoes thermal degradation and must be replaced.
Then there is the perceived safety issue. Oil-circulating systems typically use metal braided lines attached with threaded connectors and the oil pumps through the system at only 40-45 psi. Hot water usually runs through rubber hoses at 80-85 psi. These hoses are attached to the mold by quick disconnects that sometimes are not completely connected. The hazards of 93.3°C (200°F) water vs. 149°C (300°F) oil can be debated in terms of velocity and heat capacity, but the notion that higher temperature equals greater danger is firmly embedded in the minds of many processors. They simply will not employ the mold temperatures needed to properly develop the crystalline structure in high-performance, semicrystalline resins.
There are other, less obvious challenges to employing oil or electric heat. One is the problem of the time that it takes to bring the mold up to an appropriate operating temperature. Associated with this is the problem of heat transfer from the mold to the machine platens. Usually steps are necessary in mold construction to minimize this heat loss.
There is also the problem of effective heat transfer from the molten polymer to the mold. Water can be circulated at conditions sufficient to produce turbulent flow, and there are few fluids that have the heat capacity of water. Oil is not one of them, and the higher viscosity of these fluids coupled with the lower pressure will not allow for a Reynolds number high enough to achieve turbulent flow.
If electric cartridges are used, often the control setup leaves a lot to be desired. It is often simply based on a variable transformer connected to some cartridges of undefined wattage with no feedback loop from a mold thermocouple that can turn the electricity off once the mold reaches the desired temperature. And steam systems often rely on a poorly defined correlation between line pressure and mold temperature.
Detrimental losses
|
These challenges limit the number of molders who will tackle the molding of materials like PEEK and PPS. Resin suppliers know this and have gone to some lengths to ease the pain. The partially aromatic or so-called high-temperature nylons have received the most attention in this area. Virtually every supplier of these high-performance products has developed grades that can be processed using mold temperatures that can be achieved with hot water.
A casual look at the data sheets for these alternative grades does not reveal any obvious differences in performance. This is because almost all properties on a data sheet refer to room-temperature performance, and the changes to the chemistry of the polymer that are used to achieve this easier processing regimen do not substantially influence short-term room-temperature properties.
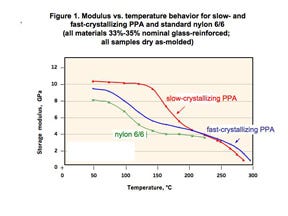
But there are sacrifices, and the differences are clearly shown in Figure 1. This shows the modulus vs. temperature behavior of a slow- and a fast-crystallizing partially aromatic polyamide along with a standard nylon 6/6. The higher-performing partially aromatic nylon requires an elevated mold temperature because it has a higher Tg.
The consequences of this higher Tg in performance are apparent. The modulus of this material is essentially constant between room temperature and approximately 140°C. However, the grade that can be molded in a water-heated tool begins to lose stiffness at about 75°C-80°C and at 140°C-150°C possesses only about 50% of the modulus of the higher-performing material. Chemically, the material supplier has manipulated the polymer structure to reduce the Tg, thereby making it possible to achieve the proper degree of crystallinity without resorting to the higher mold temperatures that oil and electric heat provide.
The differences in performance are more significant than they might first appear. A very important principle governing the behavior of viscoelastic materials is that the effects of temperature and time are equivalent for relaxation processes such as the glass transition. This means that if you want to make a quick comparison of the time-dependent behavior of two materials, simply select an application temperature on the modulus vs. temperature plots and then look at what happens as a function of increasing temperature. This behavior mirrors what will happen under load over time at that temperature. The greater the reduction in modulus that occurs with temperature, the greater the level of creep strain that can be anticipated over time.
If we select, for example, an application temperature of 70°C for the two PPA materials in Figure 1, the slow-crystallizing material will exhibit a much lower level of creep than the fast-crystallizing material. If the data sheet were the only tool available for comparing these materials, this would be virtually impossible to predict. Both materials have heat deflection temperatures of approximately 285°C and their room temperature strength and stiffness are comparable. But an examination of the temperature-dependent behavior allows us to predict that we are sacrificing long-term performance to gain ease of processing.
This is exactly what occurred with one of my clients who was using a slow-crystallizing, high-temperature nylon specifically because of a need for outstanding creep resistance at moderate temperatures. When approached with the offer of a different grade of the same product that allowed the use of water-heated molds, they changed only to discover that they had lost the creep resistance that they needed. Unfortunately, creep is long-term phenomenon, so the problems did not show up until a significant amount of product was in the field.
It is unfortunate that material suppliers will not list the advantages and disadvantages of these material choices in an objective manner. Instead, they will often minimize the downside of these easier-processing materials by highlighting small but relatively meaningless advantages in these grades. For example, if we return to Figure 1, we can see that the faster-crystallizing PPA is actually slightly stiffer at temperatures above 250°C. However, no polyamide can survive an extended period of exposure at these types of temperatures due to oxidative degradation, so the advantage has little practical value.
Rolling the dice
The other tactic that is used by material suppliers to soften the challenge of processing these high-performance materials was actually pioneered by the makers of PPS many years ago. PPS was the first significant commercial polymer that required very high mold temperatures to attain the appropriate properties. But recognizing that a lot of molders were not up to the task of heating their molds to the required temperature, PPS suppliers published processing guidelines that essentially let the molder off the hook, provided that the application did not involve exposure to elevated temperatures.
There are several concerns with sending parts out into the field that have been molded from semicrystalline materials in a manner that fails to develop the appropriate level of crystallinity. The short-term concern has to do with structural rearrangement of the undercrystallized polymer the first time it sees temperatures above the Tg. This is a process known as cold crystallization, or solid-state crystallization. It occurs in all semicrystalline polymers where quench cooling has been employed as part of the process. But the magnitude of the difference in crystallinity as a function of mold temperature is more significant for these slow-crystallizing materials.
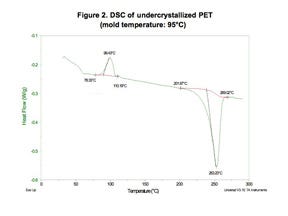
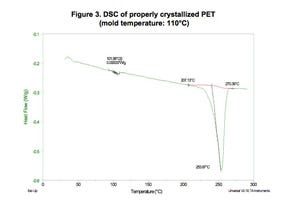
Figures 2 and 3 show DSC scans for a part molded in highly filled PET polyester at two different mold temperatures. The exothermic peak that occurs between 80°C and 110°C in the material processed at the lower mold temperature is associated with cold crystallization. These crystals should have formed during the molding process but were not able to do so because the cooling rate for the polymer was too rapid and the chain mobility required for crystal formation ceased prematurely. The absence of this peak in the part molded in the hotter mold is consistent with a properly molded part. Note that the difference in mold temperature is only 15 deg C (27 deg F).
The glass transition temperature can be observed in Figure 3 as a small step transition at 102°C.The maximum use temperature for the part is 80°C. Using the processing guidelines noted above, this is an application that should allow the molder to use the lower mold temperature because this part will not experience the elevated temperatures that could result in cold crystallization. However, in testing, the properly crystallized part exhibits significantly less creep. This results in an effective lifetime for the product that is more than 100 times longer than what was achieved with the part produced in the cooler mold.
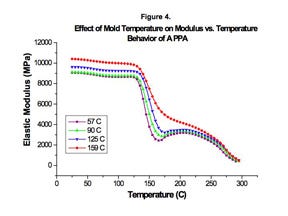
DSC shows this deficiency in an undercrystallized part as a thermal response. This is a rather abstract concept for most engineers. But dynamic mechanical analysis illustrates the consequences to mechanical performance. Figure 4 gives modulus vs. temperature plots for a high-temperature nylon molded at four different mold temperatures. Three of these plots show evidence of inadequate crystallization. Parts molded at the lower mold temperatures exhibit larger declines in modulus that begin at lower temperatures. The lower the mold temperature, the more severe is the deficiency. While this may appear only to be an issue with elevated temperature performance, recall the equivalence between temperature and time-dependent behavior. A more rapid and severe decline in modulus with temperature translates to reduced creep resistance at temperatures below the glass transition.
A study performed on glass-filled PPS showed that parts molded at 82°C (180°F) exhibited 10 times the creep strain at 80°C over the same period of time as parts molded at 140°C (284°F). This resulted in premature brittle creep rupture in the material processed in the cooler mold. In addition, studies have documented failures in undercrystallized PPS due to physical aging. This is a phenomenon that results in brittle failure and is well documented in amorphous polymers. But it is not expected in semicrystalline polymers. The occurrence of physical aging in PPS attests to the high degree of amorphous character that can be frozen into the polymer when inappropriate mold temperatures are used.
The characters in Robert Heinlein’s later novels had a single-word expression, tanstaafl, shorthand for “there is no such thing as a free lunch.” It was typically directed at those who complained that they had not been able to wrangle an undeserved reward for an inadequate level of effort. Processors would be wise to remember this simple concept. If materials are offered that ease the burden of demanding processing, they should ask the hard questions about the performance sacrifices that will almost certainly come along for the ride.
About the Author(s)
You May Also Like