The Materials Analyst, Part 116: Material validation in the early 21st century plastics industry
Even if your latest shipment of resin is what you ordered, it may not be what you need.
April 14, 2010
Even if your latest shipment of resin is what you ordered, it may not be what you need.
It is curious that in a world that relies increasingly on documentation, the attention to the details of polymer composition receive so little attention. Companies will fill volumes with procedures for various aspects of their manufacturing processes. However, no serious consideration is given to verifying the composition of the raw materials that are used to produce the various molded parts until there is a problem. Instead, there is a tendency simply to refer back to whatever published information is available from the material supplier.
|
When questioned about quality control procedures related to incoming raw materials, most processors will respond that they rely on provided certifications from the supplier. My response to this is that I have been looking at certifications for over 20 years and have yet to see one that warned me that I had just received a nonconforming batch of material.
Certifications are important because they document what the supplier says it shipped. And if all of a processor’s raw materials are provided by suppliers who have direct control over all aspects of the compound manufacturing process, then relying solely on certifications may be a low-risk approach. However, as the supply base expands to include compound suppliers who in turn purchase their feedstocks from a variety of sources, this approach becomes increasingly problematic. Lessons learned from the world of failure analysis suggest that a better approach would be to adopt the philosophy of Ronald Reagan, “Trust, but verify.”
This has proven to be even more necessary as products are sourced globally. Most of us have heard stories about products purchased from various locations around the world that turned out to be something other than what was advertised. Many of these deviations are simple to detect. A material that was supposed to contain 30% glass fiber actually contains 10%. A nylon 6 is found to be a mixture of nylon 6, nylon 6/6, polypropylene, and polyethylene. In the first case the end user notices that the material is not as rigid as expected and deflects excessively under the applied loads of the application. In the second case the material shows a wide range of problems including unmelted pellets and delamination.
The unmelt is due to the fact that the processing temperatures are set for nylon 6, a material with a melting point of 220-225°C that can be processed using melt temperatures of 235-255°C while the nylon 6/6 present in the compound melts at 260°C. There is nothing inherently wrong with blending nylon 6 and nylon 6/6, but the molder should be told about it so it can set its processing conditions accordingly. The delamination occurs because the polyolefin ingredients are inherently incompatible with nylon. While a fraction of a percent may be tolerated, when levels rise to 5%-6%, as was found in this case, the compound begins to exhibit difficulties. In both of these cases, monitoring that can cost less than $1000 caught the problems and prevented the release of a significant amount of potentially nonconforming product.
But the problem is not limited to foreign sources. With the economic pressures of the downturn still being felt, it is unfortunate but true that domestic compounders have deviated from best practices in order to cut costs to a level that goes beyond prudent. In those cases, the documentation provided with the material continues to show conformance to requirements, but the performance of the product suggests otherwise.
|
The two-year failure
The specifics of one case involved a nylon 6/6 compounded with 40% filler. The filler was composed of a mixture of short-glass fiber and a mineral. Large parts being molded from this material had shown a change in behavior over the course of a 24-month period. The product seemed to be more brittle and processed with greater difficulty. A request for certification of composition from the supplier produced a fairly detailed recipe that claimed the presence of nylon 6/6 at 57.5%, glass fiber and mineral at a combined nominal level of 40%, and “additives” comprising the remaining 2.5%.
Ash tests on parts and related raw material from two different lots, one good and one bad, confirmed a total inorganic content that ranged from 37.9%-41.2%. These values are within the acceptable industry tolerance range for a material with a nominal filler content of 40%. Because the material of more recent vintage was obviously more brittle than the material manufactured earlier in the product life cycle, molecular weight determinations were performed using intrinsic viscosity measurements. These showed that both materials had essentially the same average molecular weight. In addition, parts molded from each of these materials revealed no evidence of polymer degradation during molding.
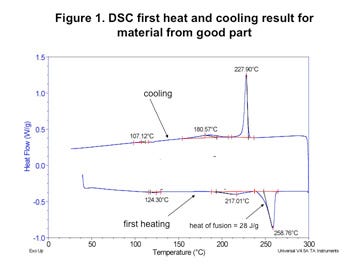
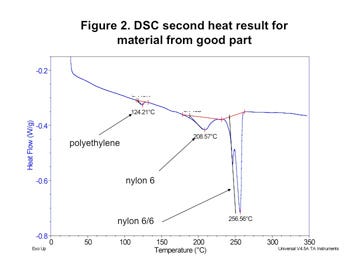
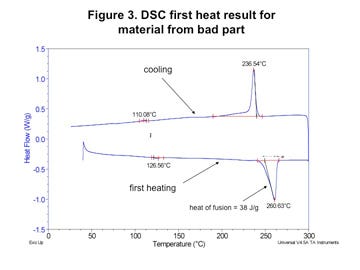
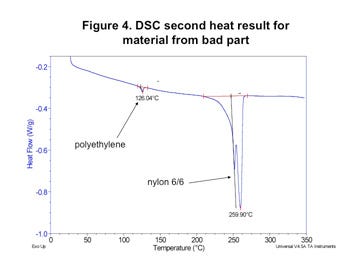
However, when the composition of the polymer was checked by DSC, substantial differences were detected. Figures 1 and 2 (left) show the test result obtained for the material that was producing good molded parts while Figures 3 and 4 (next page) provide the same test results for the material in the parts that were failing. In both tests the first graph shows the sample during initial heating through the melting point and controlled cooling back to the solid state. The second graph shows the material exposed to a second heating through the melting point.
It’s not a match
The good material shows three distinct phase changes. The smallest of these is associated with the presence of polyethylene. This is likely attributable to the carrier resin used to incorporate the black color into the base resin. While this is not an ideal practice, it is fairly common. In this case the intensity of the melting event indicates that the polyethylene makes up no more than 0.5% of the compound. Approximately the same level was determined to be present in both materials.
The largest melting transition in both scans is attributable to nylon 6/6. On first heating this appears as a single peak and on second heat it is split into two distinct but overlapping peaks. This is characteristic behavior for nylon 6/6. However, the energy associated with the melting of this polymer is much higher in the bad material; 38 J/g as opposed to 28 J/g for the good material. This can be explained by the presence of the third melting event in the good material. This event, which peaks at 217°C, is consistent with nylon 6.
The presence of the nylon 6 produces several changes in the melting behavior of the material. First, the melting point of the nylon 6/6 phase in the good material is 2-3 deg C lower than it is in the bad material. This melting point suppression is typical in nylon 6/6 that has been mixed with nylon 6 and then repeatedly melted and recrystallized. In addition, the melting point of the nylon 6 polymer is also suppressed. Normally, pure nylon 6 melts at 220-225°C. While the initial scan of the as-received material produces a melting point of 217°C, the slow heating and cooling process associated with the DSC test produces a further suppression of the melting point to 208.6°C.
These differences carry over to the cooling portion of the test cycle as well. The nylon 6 exhibits a distinct recrystallization with a peak just above 180°C and the recrystallization peak for the nylon 6/6 portion of the compound is suppressed nearly 9 deg C, another effect of mixing the two nylon polymers. The strength of the melting endotherm associated with the nylon 6 shows that it is present at a substantial concentration.
Strict quantification of nylon 6 and nylon 6/6 in a mixture can be a tricky proposition because of the influence that the two polymers have on each other. However, the strength of the melting endotherms allows for at least an estimate of the nylon 6 concentration at 15%-20%. While the minor amount of polyethylene in each material can be classified as part of the “additive” line item, the nylon 6 levels go well beyond this point and represent a significant deviation from the recipe in the certification. To be blunt, the actual composition of the material in this sample does not match the documentation.
Documentation vs. 
competence
The irony of this situation is that the material that deviates from the specification is the material that performs well. The product that was found to be a problem actually matches the certification and the published data sheet. Had the processor or the end user devoted the resources to verification of composition at the beginning of the program, this deviation could have been identified and reviewed prior to ever going into production.
In addition, the change in material composition represented by the two materials shows that there was likely no program of incoming inspection for materials being supplied to the compounder. So despite the presence of an impressive array of documents, unauthorized and undetected changes were made to the material that resulted in the production of a substantial amount of nonconforming product.
John Ruskin, the 19th century English social thinker, observed, “There is hardly anything in the world that some man cannot make a little worse and sell a little cheaper, and the people who consider price only are this man’s lawful prey.” Benicio Del Toro’s character, Mr. Longbaugh, put it in more colorful terms when he said, “There is always free cheese in a mousetrap.” In these cases the mousetrap is populated with product recalls, customer dissatisfaction, lawsuits, and all the associated distractions that take us away from doing the business that our companies were established to conduct. In the final analysis, documentation is no substitute for trust and competence.
About the Author(s)
You May Also Like