The Materials Analyst, Part 98: A tour of the world of nylon—Part 1
July 1, 2008
|
With so many similarities between nylon 6 and 6/6 at a molecular level, evaluating bonds and subtle structural shifts explain how small differences can have a big impact on the final product.
This is another one of those pieces prompted by an unscientific piece of advice (actually two unscientific pieces of advice) given to one of my clients. It started with an e-mail asking why it was that my client's caster supplier could not produce casters from nylon 6/6 when they were already producing them from nylon 6. The supplier's response was that “their machines and tools are not conducive to processing the 6/6 material.” No particular reason was given.
When my client went looking for a little help with this puzzle, a friend of his advised him that the two materials were virtually the same and that the only real difference was that nylon 6 was created by “changing/shifting one atom to the left” to get around patent issues.
This is one of those statements that, while not entirely accurate, has a kernel of truth at the center. The key to understanding the differences and similarities between nylon 6 and nylon 6/6 starts with knowing what the numbers mean. But while we're at it, let's take a tour of the entire nylon commercial realm. It is becoming a crowded field with so-called “low-moisture-absorbing nylons” (nylon 6/10, 6/12, 11, and 12), high-temperature nylons, transparent nylons, and even a high-performance compound that cannot be melt processed known as aramid but more familiar to many under the trade name of Kevlar.
|
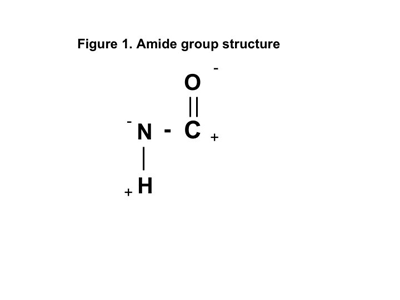
Since nylon 6 and nylon 6/6 are the most commercially successful compounds in the family, we will use these materials to come to an understanding of what has to be present in a material to qualify it for membership in the nylon pantheon. At the heart of the nylon chemistry is a chemical constituent known as an amide group. The structure of this group is shown in Figure 1. Notice the plus and minus signs next to the elements in the group. These will be important as our discussion progresses.
THE CREATION AND NAMING OF NYLON
Polymer names often grow out of the monomers from which they are made. Ethylene becomes polyethylene, styrene becomes polystyrene, and so on. Or they adopt a shorthand from some distinguishing characteristic within the chemistry. For example, polyesters contain a distinct chemical group known as an ester group, and you can find a carbonate group recurring in polycarbonate. Most of the world, therefore, refers to nylons as polyamides.
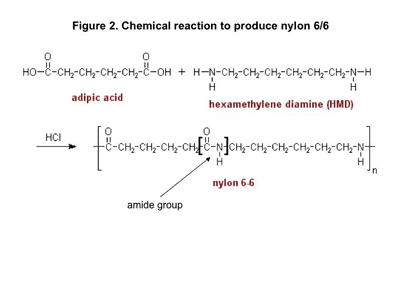
The name nylon was assigned to the first successfully synthesized version of the polymer by the executives at DuPont and there are many apocryphal stories about how the name was selected. Wallace Carothers, a chemist working at DuPont, is credited with creating a product that was made by reacting together two chemicals, adipic acid and hexamethylene diamine.
The reaction is shown in Figure 2. Notice that both the reactants are made up of short chains that each contain six carbon atoms. Hence the name nylon 6/6, also sometimes written as nylon 66. Carothers derived his inspiration from his studies of silk, a natural substance with similar chemistry.
Without going into too many of the details, the acid contributes the C=O functionality and the amine contributes the N-H group to what becomes the amide group. This is highlighted in the structural repeating unit. A single nylon chain may contain 100-150 of these repeating units. What is not shown here is that a water molecule is given off as a byproduct when this reaction occurs. Consequently, chemists classify this as a condensation reaction and refer to nylon 6/6 as a condensation polymer. We will come back to this in the next article.
This amide group has some remarkable qualities that account for the balance of properties that nylons as a family provide. The central feature of the amide group is its ability to form something called a hydrogen bond. To explain hydrogen bonding we need to take a moment to briefly discuss the types of bonds that develop when atoms are brought together to form compounds.
The bonds within the molecules that make up a compound are comparatively strong. These bonds represent a sharing of a pair of electrons. Both atoms contribute an electron to this collaboration and the result is a very strong connection between the atoms. In organic compounds such as polymers, these are typically covalent bonds such as those that connect carbon atoms to each other (C-C) or that connect a carbon atom to a hydrogen atom (C-H). These covalent bonds are depicted in chemical structures by the single and double lines drawn between the atoms in the molecules. A great deal of energy is required to break these bonds, which is a good thing because these are the bonds that hold the molecules together. These molecules define the properties of the compound.
However, some of the properties are determined not by the bonds within the molecule but by the bonds between the molecules. For example, the energy required to separate the molecules in a crystal lattice from one another and get them sliding around past one another determines the melting point of the material. The energy required to completely separate the molecules from one another and allow them to go flying off in different directions governs the boiling point. These phase change temperatures are a measure of the attractive forces between the molecules. The strength of these attractions is, in turn, a function of the types of atoms that are bonded to each other within the molecules.
NONPOLAR MATERIALS
We recognize two distinct groups of compounds in this respect. Those made up of atoms that share their electrons in a more or less equitable fashion are referred to as nonpolar materials. The distribution of electrons is so even throughout the molecules that there is no sustained dislocation of electrical charge. This results in attractions between the molecules that are very weak.
One example of this is gases that are made up of two atoms of the same element, such as hydrogen, oxygen, or nitrogen. These materials are gases at room temperature because the energy that each molecule possesses causes them to move at a velocity that overcomes the relatively weak attractive forces between them. To liquefy these materials we need to slow down the molecules significantly, and this is done by reducing the temperature. We have to reduce the temperature to more than 200°C (360°F) below room temperature to turn oxygen and nitrogen into liquids.
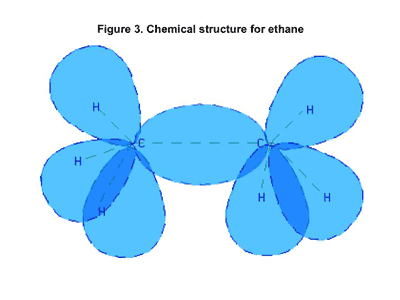
Another example of nonpolar compounds is a family of materials known as hydrocarbons. These are materials made up of only carbon and hydrogen. Figure 3 shows a schematic of the bonding in an ethane molecule. These atoms share their electrons very evenly and therefore these materials are also nonpolar. The smallest hydrocarbon is methane, and it has a boiling point that is only slightly above that of oxygen and nitrogen. But temperature is a measure of kinetic energy, and this energy of motion is a function of the velocity of the molecule and its mass. So at a given temperature, heavier molecules move more slowly than lighter ones.
The next heaviest hydrocarbon in the series to which methane belongs is ethane, followed by propane and butane. All are gases at room temperature. But the next two materials in this series, pentane and hexane, can exist as liquids at room temperature. And by the time we get to the molecule in this series with 20 carbon atoms, we have reached the point where the molecules stay in fixed positions at room temperature and we have a solid.
Table 1 shows some of the molecules in this chemical series and their melting and boiling points. The last member in this table is a polymer that is actually a very large version of the smaller nonpolar molecules. It is polyethylene, and in the high-density variety it has a melting point of about 135°C.
We have come a long way from the melting point of methane without changing the nature of the intermolecular attraction. We accomplished this simply by increasing the size of the molecules. But in the universe of polymeric materials, polyethylene is not known as a heat-resistant material, primarily because the attractive forces between the molecules are relatively weak and can be broken with relatively modest increases in temperature.
POLAR MATERIALS
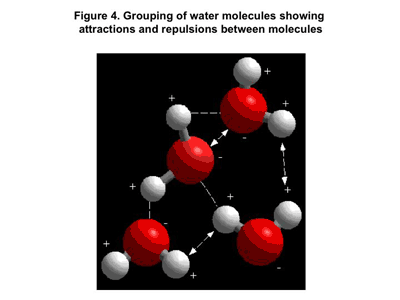
The other family of materials is referred to as polar. The molecules in these materials are formed from atoms that have very different attractive tendencies regarding the shared electron pair. A good example of this type of compound is water. Water forms by combining two hydrogen atoms with one oxygen atom. An electron pair is shared between the oxygen atom and each hydrogen atom. But in this case the sharing is unequal. The electrons actually spend much more of their time close to the oxygen atom, and this creates a dipole, a little magnet with a distinct positive and negative end or north and south pole. Neighboring water molecules tend to line up the way magnets naturally would, with the north or positive end of one molecule oriented toward the south or negative end of the next molecule.
Figure 4 shows a grouping of water molecules. This polarity results in much stronger attractions between the molecules and we would expect that the melting and boiling point of water would be much higher than that of a hydrocarbon of comparable mass. We would be correct. But there is more going on here than just simple polarity. Hydrogen is unique in that it is the only element capable of forming compounds that has only one shell or layer of electrons surrounding the positively charged nucleus of the atom. So when the electron is removed by a strongly attractive atomic partner such as oxygen or nitrogen, the positively charged nucleus is exposed. This causes the attractive forces between the molecules to be especially strong and this special case has a special name. We call it a hydrogen bond. It is the strongest of all the polar attractions between molecules.
What does this additional attractive force buy us? Well, water molecules are of approximately the same mass as methane molecules. Based solely on this consideration, we would expect that methane and water would have approximately the same melting and boiling points. However, the melting point of water is 0°C (32°F), almost 183 deg C (330 deg F) higher than that of methane. And the boiling point of water is 100°C (212°F), 262 deg C (472 deg F) above that of methane. So what would we expect from a polymer that contained these same hydrogen bonds?
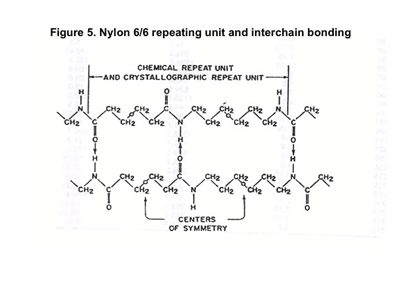
Figure 5 shows the repeating unit structure for nylon 6/6. The material exhibits a repeating unit that contains the amide groups separated by methylene (-CH2-) groups spaced in a 4-6-4-6 pattern. These are the same methylene groups that make up the entire polymer chain in polyethylene. They are nonpolar, loosely attached, and free to rotate. But the polar points in the nylon chain are tightly bonded to the groups in neighboring chain segments, forming the same strong attractions we saw in water.
This is the significance of the plus and minus symbols in Figure 1. There is a natural strong attraction between the negative oxygen end of the C=O group on one chain segment and the positive hydrogen end of the N-H on the neighboring chain segment. The symmetry of the 4-6 pattern provides an excellent opportunity for the polar groups to align, creating hydrogen bonding within the crystalline regions of the nylon 6/6.
THE DIFFERENCE OF A SUBTLE SHIFT
With DuPont holding patents on the process for making this material, other researchers sought ways to create a similar material by different routes. BASF was particularly interested in polyamides and found that by working with ring-shaped structures that contained both ends of the amide structure in the same molecule, it could produce compounds with similar properties.
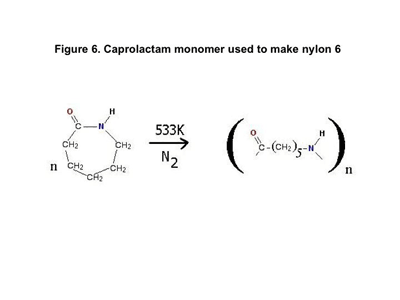
Figure 6 shows the reaction that is used to produce nylon 6. It begins with a single monomer known generically as caprolactam, a compound with six carbon atoms in the ring. By breaking the carbon-nitrogen bond, the ring is opened and the monomer can react with itself to produce the polymer we know today as nylon 6.
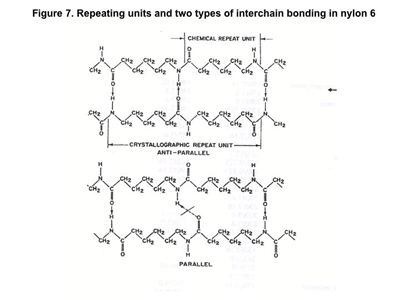
You can see where someone would form the opinion that the two materials are essentially the same with “one atom shifted to the left.” But it is not quite that simple. The amide group in nylon occurs every five methylene groups. Statistically, therefore, the average spacing of amide groups in nylon 6 and nylon 6/6 is the same. But Figure 7 shows two ways in which the nylon 6 polymer chains align. One provides the same symmetry and opportunity for polar alignment as in nylon 6/6. But the other creates a situation where the distance between the polar groups is too great to always allow for the closest possible spacing.
If the hydrogen bond is so instrumental in determining phase change temperatures, we would expect that this subtle shift in symmetry would be detectable in something like the melting point of the two materials. And this is the case. Nylon 6/6 has a crystalline melting point of 260-265°C while the melting point for nylon 6 is 220-225°C. Both of these are substantially higher than that of HDPE, which can be thought of as nylon without its polar groups, but the slight change in the molecular arrangement is not trivial, particularly when it comes to short-term heat resistance. The increased opportunity for molecular alignment also means that nylon 6/6 crystallizes faster when it cools and potentially can form more crystals, making it a slightly stronger, stiffer material.
We can make other types of polyamides by changing the chemicals that we use to make the polymer. This changes the spacing of the amide groups. Closer spacing increases the melting point, strength, stiffness, and density of the polymer as in nylon 4/6. Greater spacing will decrease all these properties because the opportunity for hydrogen bonding declines. This relationship is shown in Table 2. In fact, Carothers, at the time he invented nylon 6/6, was also working on a reaction between pentamethylene diamine (five carbons) and sebacic acid (10 carbons) that actually produced a polymer with better properties than nylon 6/6. But it was too expensive to manufacture. It would have been called nylon 5/10.
There is another important consequence of the presence of the amide group. Because the amide group and water both contain the potential for hydrogen bonding, amide groups that do not bond to each other will potentially attract water molecules. This accounts for the very hygroscopic character of polyamides, a complicating property that all processors and users of nylon materials must cope with.
In the next article we will discuss this property, talk about some of the strategies used to manage it, and highlight some newer variations in nylons that extend the utility of this old polymer family. We will also look more closely at why molds and machines running nylon 6 would be incapable of processing nylon 6/6.
About the Author(s)
You May Also Like