"Magic" wand that enables remote medical exams showcased at consumer electronics show
Once touted as the next big thing in healthcare, telemedicine mostly has failed to live up to its potential. Regulatory obstacles are one reason: In many cases, physicians cannot practice in multiple states, for example, and one of the raisons d'être of telemedicine, which encompasses everything from remote consultations via webcams to self-monitoring, is the erasure of spatial and geographic constraints in healthcare. It's also true that practitioners, a conservative bunch, can be reluctant to embrace a technology that would reduce office visits.
January 4, 2016

of telemedicine, which encompasses everything from remote consultations via webcams to self-monitoring, is the erasure of spatial and geographic constraints in healthcare. It's also true that practitioners, a conservative bunch, can be reluctant to embrace a technology that would reduce office visits. The paucity of breakthrough applications that leverage the technology in ways that excite the public imagination has also contributed to the low profile of telemedicine. That may change, however, with the introduction of the MedWand, described as the world's first handheld device that combines several diagnostic tools for use with remotely located medical providers. The product is being demoed at CES 2016, the vast consumer electronics show that invades Las Vegas this week.
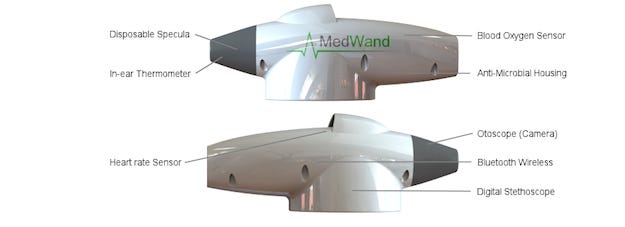
Somewhat reminiscent of the Tricorder of Star Trek fame, MedWand enables remote examinations by incorporating a pulse oximeter; an otoscope camera for ear examinations with attachments to also allow views of the eyes, throat or nose; an in-ear thermometer; a digital stethoscope; and the capability to support third-party Bluetooth wireless devices such as glucose meters or blood pressure monitors. All of the diagnostic data can be bundled into a secure Electronic Patient Record, enabling a clinical-quality, interactive, at-home telemedicine experience for patients and their doctors, says the company.
"Telemedicine finds its disruptive technology," noted one recent headline. CES attendees will be able to judge for themselves, as Dr. Samir Qamar, MedWand creator and the tech startup's CEO, performs live demonstrations.
For many industry observers, however, the verdict is already in. MedWand recently received the Health 2.0 Launch award, beating out nine other medtech startups in the prestigious program that crowns advances in new health technologies. The device also was named one of the top 10 disruptors in healthcare in 2015 by Frost & Sullivan.
MedWand Digital Health expects to receive FDA clearance for its signature product this year; a commercial roll out will follow shortly after that, but CES attendees will be among the first to see the finished device. "We're going to debut MedWand's new design, the one consumers will receive when we go into production," said Lead Engineer and President Robert Rose.
Home and clinical MedWand packages can be preordered on the company's website. A standalone MedWand for home use that functions with a tablet, PC or Mac costs $249; the MedWand Home Clinic package, which comes with a tablet PC preloaded with all of the necessary software, retails for $695.
About the Author(s)
You May Also Like




