October 12, 2022
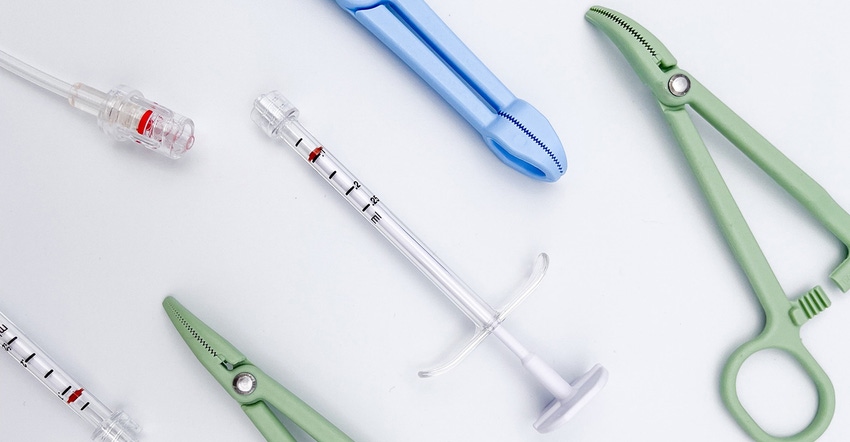
Ascend Performance Materials has launched a new portfolio of medical-grade nylon 6,6 resins and engineered materials for the healthcare market. The HiDura Med products meet ISO 10993-5 and 10993-10 biocompatibility testing criteria and can be used in a variety of healthcare applications.
“Ascend’s nylon 6,6 brands are synonymous with unparalleled quality across multiple industry segments,” said Dhruv Shah, Ascend’s healthcare business manager. “Our HiDura Med portfolio offers the same quality to customers looking for solutions that meet the stringent requirements of the healthcare market, including ISO 10993-5 and 10993-10 biocompatibility testing, an effective change notification policy, and operational policies to ensure the highest possible standards.”
HiDura Med applications include:
Braces, patient support, furniture, mobility aids, and other durable equipment;
drug-delivery systems (filtration equipment and membranes, tubing, fluid connectors, and auto injectors);
surgical instruments;
housings, protective cases, cables, sensors, connectors, and wearables;
wound-care products such as sutures, tapes, and zip ties.
Ascend Performance Materials said it will continue to expand its healthcare portfolio, and plans to introduce long-chain and amorphous polyamide grades in the future. Ascend is also actively exploring applications in medical durables and wound care using Acteev, its patented technology that incorporates the antimicrobial benefits of active zinc ions in polyamide to minimize growth of microbes that can cause some medical products to degrade.
You May Also Like


