July 12, 2023
A new type of synthetic heart valve could soon be available to help treat children living with chronic rheumatic heart disease. Researchers at Harvard University’s Wyss Institute and John A. Paulson School of Engineering and Applied Sciences (SEAS) are reportedly closer to releasing their FibraValve, a next-generation implantable heart valve that is anticipated to reduce the number of invasive surgeries typically required for kids growing up with pediatric heart valve disease. The condition typically affects older individuals but it can also be congenital or brought on by infections such as strep throat when treatment is not ideal.
The spun-fiber valve can be manufactured in less than 10 minutes by utilizing a new method known as focused rotary jet spinning (FRJS), a process that allows the valve’s delicate flaps to have customizable shapes and properties down to the nanoscale.
According to a recent study published in the peer-reviewed journal Matter, FibraValve was readily colonized by living cells in vitro and in large animal model studies conducted by collaborators led by the Wyss Zurich Translational Center in Switzerland.
“This study illustrates FibraValve’s potential as a solution for children suffering from valve diseases,” said Kit Parker, PhD, associate faculty member at the Wyss Institute and the Tarr Family Professor of Bioengineering and Applied Physics at SEAS, in a press release issued by Wyss Institute officials. “Our goal is for the patient’s native cells to use the device as a blueprint to regenerate their own living valve tissue, but FRJS also has potential as a platform to fabricate other medical devices in the future.”
The jet (spinning) set
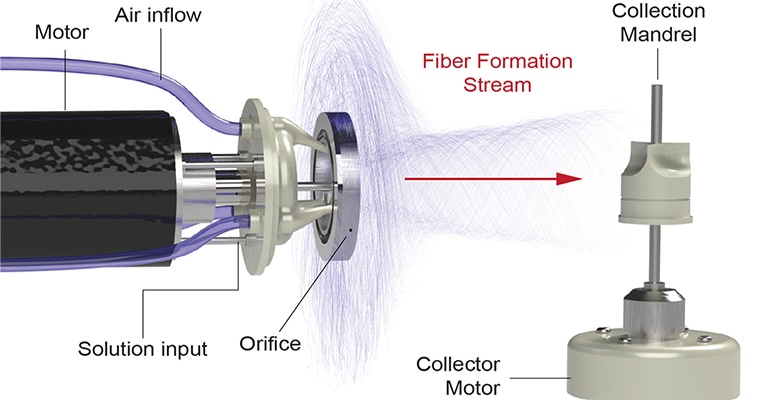
A follow-up to the institute’s first synthetic heart valve — the JetValve, the first device manufactured using the nanofiber fabrication technique FRJS — FibraValve has been in development since 2014, according to Wyss officials. The FRJS technique extrudes a biocompatible synthetic polymer solution into long fibers that wrap themselves around a mandrel shaped like a heart valve. As Wyss officials describe it, the FRJS process is similar to how a cotton candy machine spins sugar crystals into the fluffy substance that’s wrapped around a paper cone.
|
Focused rotary jet spinning produces long fibers from a biocompatible synthetic polymer solution that wrap themselves around a mandrel. The process can be be compared to a cotton candy machine. |
The JetValve previously has been successfully implanted into a sheep’s heart using minimally invasive surgery, recruiting living cells to regenerate new tissue on the device. Building on this approach, researchers have reportedly re-visited multiple aspects of JetValve manufacturing, fiber material, and geometric design as they have worked on the FibraValve.
Polymer fibers mimic heart valve's tissue structure
For the manufacturing process, streams of focused air are added to the polymer-extruding stream to create FRJS. This has allowed for improvements in the rate that polymer fibers are deposited onto the mandrel and in the ability to adjust the resulting valve to a final desired shape. The polymer also produces networks of microfibers and nanofibers that better mimic the tissue structure of a human heart valve.
A newly customized polymer material known as PLCL has also been used to improve the infiltration of living cells once the FibraValve is implanted. A combination of polycaprolactone (PCL) and polylactic acid (PLA), PLCL has been shown in previous studies to last approximately six months after implantation in rats. According to Wyss officials, PLCL is infiltrated by living cells, remodeled into functional tissue, and is safely biodegradable. FibraValve reportedly also enables a more even distribution of cells throughout its scaffold than valves made of other polymers.
The geometry of the FibraValve is said to be optimized through the shape of the valve’s inner “leaflets” to minimize leakage of blood backwards through the valve. Use of the valve reportedly reduced the amount of leakage from ~30% with the JetValve to ~13% with the newly designed FibraValve.
Recent results, future testing
After implantation into a living sheep’s heart using minimally invasive surgery, FibraValve reportedly began to function immediately, with red and white blood cells infiltrating the valve’s porous scaffolding, and fibrin protein being deposited on the outside of the valve, within one hour. The valve also displayed no damage or problems following implantation. Longer-term animal testing to evaluate FibraValve’s performance and regenerative capabilities over weeks and months is anticipated to occur soon. Other potential implantable devices with FRJS could also include cardiac patches and blood vessels.
“This approach to heart valve replacement might open the door toward customized medical implants that regenerate and grow with the patient, making children’s lives better,” said Michael Peters, co-first author of the recent study and a graduate student at the Wyss Institute and SEAS.
About the Author(s)
You May Also Like