FDA Investigates Failure-Prone Plastic Syringes Made in China
Agency recommends avoiding use of plastic syringes manufactured in China as it evaluates quality issues resulting in leakage and breakage.
December 4, 2023
At a Glance
- FDA has received information about quality issues associated with several China-based manufacturers of syringes
- Manufacturers made changes to syringe dimensions, affecting performance and safety
- BD syringes not affected as almost all are made in USA
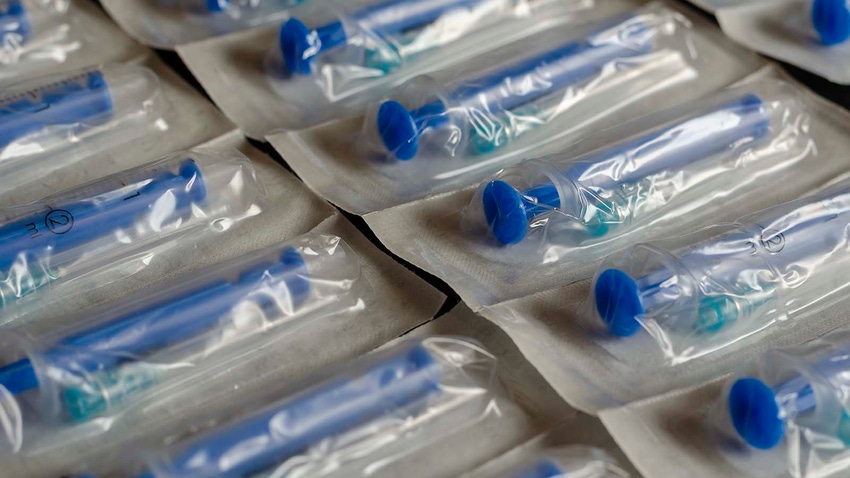
FDA reported on Nov. 30 that it is evaluating plastic medical syringes made in China for potential device failures, such as leakage and breakage.
The agency said in a statement issued on Thursday that it has received information about quality issues associated with several China-based manufacturers of syringes. It is currently collecting and analyzing data on plastic syringes made in China that are used to inject or withdraw fluids into and from the body. The investigation does not involve glass or pre-filled syringes or those used for oral or topical applications, FDA noted.
The investigation was launched as FDA became aware of quality issues related to recent syringe recalls, medical device reports, and various complaints. “Quality issues reported have included leaks, breakage, and other problems after manufacturers made changes to the syringe dimensions. These quality issues may affect the performance and safety of the syringes including their ability to deliver the correct dose of medication when used alone or with other medical devices such as infusion pumps,” said FDA in the announcement.
While it conducts its evaluation, FDA recommends that Chinese-made syringes that fit this description not be used. “If you only have syringes manufactured in China, then continue to use them as needed until you are able to use alternative syringes and closely monitor for leaks, breakage, and other problems,” the agency added.
Medtech giant BD immediately followed up on the safety notice by posting a press release on its website stating that this does not apply to any of its syringes. “Essentially all plastic syringes BD provides to the US healthcare system are manufactured in the United States in Nebraska and Connecticut,” said BD Medication Delivery Solutions President Eric Borin. "BD . . . is ready to increase production to help supply those providers who currently purchase syringes impacted by the FDA communication,” he added.
Likewise, the world’s largest manufacturer of plastic insulin syringes, embecta, said that it manufactures all of its syringes at a facility in Nebraska and, therefore, is not affected by the FDA safety communication.
About the Author(s)
You May Also Like




