Material innovations aid medical design
New design trends and increasing health, environmental, and safety concerns for medical products can be addressed with a wide variety of new materials on the market.
April 23, 2009
New design trends and increasing health, environmental, and safety concerns for medical products can be addressed with a wide variety of new materials on the market.
While many molding end markets are struggling, one that is relatively thriving is medical. Unlike declining automotive sales, people will always need medical care, even in a recession; and the medical device manufacturing industry is estimated at $146 billion and growing. Many material suppliers have been hard at work, anticipating upcoming regulations and creating compliant grades free of phalates, bisphenol A (BPA), and halogens. Several innovative products were introduced at the colocated Plastec West and MD&M West shows, held Feb. 10-12 in Anaheim, CA, organized by IMM and MPW parent Canon Communications LLC.
|
Among the medical material experts exhibiting at the show, many mentioned the expanding need for aesthetic yet functional design and materials for home healthcare devices, as well as properties desired for clarity, lipid resistance, and resistance to bacteria. With a minimum product development cycle of 18 months due to FDA regulations and validation, most of these new materials will likely be used in new designs. But with the ever-expanding selection of materials to choose from, finding the perfect material for a potential customer’s products can be a difficult task.
Josh Blackmore, global market manager healthcare for RTP Co. (Winona, MN), believes that every good design begins with market studies to determine trends in design. There has been a recent crossover of medical devices in the home, making aesthetically pleasing yet durable designs more important. Molded-in colors that look like paint have been a trend in this area, as well as using softer, squishier materials to aid in the grip for device insertion, for example. For overmolded gripping areas or IV components, durometers of 60-95 A are becoming more popular, as is including colorful brand logos, even on single-use disposables.
Healthcare heads home
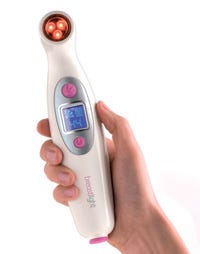
As the healthcare industry puts a greater emphasis on preventive health and the use of in-home devices, designers must consider durability and appearance more than ever. When start-up life sciences company PWB Health Ltd. (Dumbarton, Scotland) wanted to bring a home-use device for breast cancer detection self-examinations to the market quickly, the company paired with Sabic Innovative Plastics (Pittsfield, MA) for technical and design services. “We worked very early on with PWB and designer Wideblue to determine where it was going to be used,” says Tom O’Brien, industry manager healthcare for Sabic. “We like working with designers for home healthcare trends, which are now more like consumer trends.”
The Breastlight device shines a light on breast tissue, illuminating internal changes, so the lens required a crystal-clear material with excellent impact resistance and the ability to be ultrasonically welded. Lexan polycarbonate met these needs, and the grade selected is also assessed to the ISO 10993 standard for biocompatibility.
For the housing, impact resistance was a concern, since the device would likely be used in a bathroom with a tiled floor, and PWB wanted it to be lightweight, which meant a high-flow resin would be required for the thin walls. It also had to minimize light seepage from the high-power red LED bulbs while remaining the specified white color. A healthcare grade of Cycoloy PC/ABS was selected, and the desired white shade was color-matched at Sabic’s ColorXpress facility in Bergen op Zoom, the Netherlands.
The Breastlight is currently under review by the FDA for sale in the United States and has been approved to sell in Europe and Canada. Wideblue (Dumbarton, Scotland), PWB Health’s design and development partner, won the breakthrough product Innovation & Design Excellence Award (IDEA) in 2008 for its work on the Breastlight, which was exhibited for the first time in the U.S. at Sabic’s booth at Plastec West.
Eco-conscious options
A shift toward evaluating the environmental impact of plastics has led medical device designers and manufacturers away from traditional polymers that may incorporate halogens, phthalate-based plasticizers, or high levels of extractables. In order to provide more eco-conscious material choices, GLS Thermoplastic Elastomers (McHenry, IL) introduced a portfolio of FDA-compliant, recyclable, medical-grade TPEs at Plastec West.
“There’s currently a lot of discussion about the health risks of phthalates and other plastic additives, and although the jury is still out, the clear trend is for increasing restrictions in the use of these substances,” says Joe Kutka, technology launch manager for GLS. “Added to this is the mandate to create products that can be recycled or, alternatively, incinerated or disposed of without releasing halogens.”
Three grades of the company’s Versaflex HC in 85, 90, and 95 Shore A hardnesses offer high clarity and flexibility without plasticizers or halogens, while providing stability under various sterilization methods. The other two grades are translucent and resistant to high temperatures, with the 68 Shore A version able to be autoclaved at up to 275°F, and the 57 Shore A grade resistant to 260°F. Medical tubing is an ideal application for these materials, but since all grades can be injection molded or extruded, manufacturers have the option to use a single material for both tubing and molded connectors.
For designers looking for a strong, clear, lipid-resistant material free of halogen and BPA, Eastman Chemical Co. (Kingsport, TN) introduced a medical grade of Tritan copolyester. The copolyester is easy to process (unlike PC grades modified for increased lipid resistance), has a minimum color shift after gamma sterilization, and is much stronger than acrylic. Two grades were introduced at Plastec West, one neat and one with a mold-release agent compounded in, and the material is said to be ideal for IV system components, respiratory devices, and blood therapy devices.
“We’re uniquely positioned with copolyester,” says Scott Hanson, global industry leader medical for Eastman. “Our competition is the acrylics and polycarbonates. The balance of properties is the reason we developed the Tritan medical grade, not the fact that it’s free of BPA, though it is another benefit.”
Silicone and antimicrobial advancements
With the tactile feel of medical devices becoming more of a design element, it’s no surprise that new material advances have been made in liquid silicone rubbers (LSR). “The LSR 7000 platinum-catalyzed LSR series was originally introduced for potential use in specialty electronics applications, but its high optical transparency and excellent physical stability make it an ideal product to consider for healthcare in applications such as wound drains, endoscopic devices, catheters, and respiratory components,“ says Lynn Colucci-Mizenko, global marketing manager healthcare for Momentive Performance Materials (Aurora, IL).
Introduced at Plastec West, the product is used in a 1:1 mix ratio and typically cures rapidly to a high-strength elastomer by heating in standard injection molding equipment. The high temperature stability of this addition-cured LSR can also be considered when potential stress cracking of thermoplastics is a concern.
According to Colucci-Mizenko, an antimicrobial additive that can be added to Momentive’s LSR and HCE base elastomers has been experiencing a great response from the healthcare market since its introduction at MD&M East in New York last summer. The Statsil additive uses the positive charge carried by ionic silver to alter negatively charged groups of biological molecules, resulting in inhibition of bacterial and microbial growth. Ideal for applications such as catheters, wound drains, and IV components, Statsil requires low additive loading levels, which disperse throughout the polymer matrix and won’t abrade off, for longer-lasting effects.
Another product line extension also uses antimicrobial technology to fight against methicillin-resistant staphylococcus aureus (MRSA) and other clinical infections associated with medical devices. At the Plastec show, AdvanSource Biomaterials (Wilmington, MA) added antimicrobial extension lines that complement the company’s ChronoFlex and HydroMed polyurethane products. The full homogenous dispersion throughout the polymer is said to provide a 99% kill rate of the MRSA bacteria and is less susceptible to bacterial growth and biofilm formations.
In addition to antimicrobial properties from the additive, these materials were developed to overcome a wide range of design and functional challenges, including the need for dimensional stability, ease of manufacturing, and providing heightened lubricity for ease of insertion.
Use your resources wisely
So, how does one know where to begin with so many options? When finding a material supplier, ask what value-added design and technical services are available. Since many suppliers have separate medical material divisions, chances are that products requiring similar properties have been dealt with before, and the experience provided by these materials experts could potentially help shorten the product development cycle.
But the extensive information available on each potential material’s chemical resistance, physical properties, and processing parameters can be overwhelming. To help designers with material selection, Teknor Apex Co. (Pawtucket, RI) introduced a resource guide at Plastec West (which can be requested at www.medalistmd.com) on each of the company’s 33 Medalist elastomer compounds, including a grade selector and tabbed sections for process guidelines, design considerations, and physical properties. Asking material suppliers for the latest information on new grades can help raise awareness for new capabilities designers can add to products.
With a little patience and some educated material selection, shifting business toward the highly regulated medical industry doesn’t have to be difficult. Good ideas can turn into products quickly, and the advances and information available from medical material suppliers can help get these products to market quickly. —[email protected]
About the Author(s)
You May Also Like