Medical Marvels Coming in 2023, Made Possible by Plastics 50804
Tremendous technological advances are leaving the confines of R&D to meet global healthcare needs.
January 3, 2023

Len Czuba
We live in interesting times! This (almost) post-pandemic era is just now allowing the unfolding of new technology that was being worked on but not introduced to the industry while much of the world was in lock-down. Get ready to hear about some of the latest and greatest new developments that will help advance healthcare for all in 2023!
The COVID-19 virus and resultant worldwide pandemic hit the citizenry of the world severely! Millions died and 10-times that number became sick with the virus. I have been impressed by how rapidly the biotech industry was able to come up with diagnostic tests to detect the virus in patients and how quickly the bio-pharmaceutical industry was able to develop vaccines. In both cases, the medical plastics industry served to help make personal protective equipment, test swabs, kits, and ultimately the vaccines that helped bring resistance to the virus. As the threat of severe viral effects are reduced and mostly eliminated, the diagnostics, bio-pharma, and medical device communities are now able to focus on other areas of need.
Advances in diagnostics
In diagnostics, we see products such as continuous glucose monitors (CGMs) that help diabetic patients more closely monitor and control their blood-glucose levels and avoid the complications that come with frequent extreme high and low glucose levels. See Dexcom’s G7 and Abbott’s FreeStyle Libre. The latest CGMs almost completely eliminate the need for frequent needle-sticks to test blood-glucose levels. Soon to be developed and introduced are systems that will act together to automatically administer insulin-type medication when high blood-glucose levels are detected. New technologies in transdermal sensors, skin adhesives, and electronics that enable the monitoring all work together to make these CGMs a reality. Also in development are implantable systems, such as TheraCyte, which support living cells implanted into patients that will generate the needed insulin and be self-regulating, leading to needle-free treatment!
|
Next-gen continuous glucose monitors, such as Dexcom's G7, bring tangible quality-of-life improvements to patients. |
The bio-pharmaceutical industry has benefitted from single-use systems (SUS) in the development of new vaccine drugs and, once developed, in the production of large-volume batches of the novel vaccines. SUS have reduced development time and the cost of new drug research. And when the new drug moves into production, better control and lower costs help speed up production, enabling distribution of the medicines throughout the world. SUS suppliers such as ThermoFisher and Qosina have resources available to those needing selection recommendations.
Connected textiles
In much the same way, medical textiles are being developed and refined that have built-in sensor technology, antimicrobial features, signal detection, and the ability to monitor health signals on a continuous basis. These textiles will be linked to connected devices to communicate directly with the healthcare providers not only to monitor but also treat the patient, as needed. The early treatment of a health condition will help prevent its escalation into a severe condition such as a heart attack or stroke.
Novel polymers are being developed that can deliver active pharmaceutical ingredients (APIs) or medication directly to the patient as needed, often over extended time periods. Leistritz twin-screw compounding systems are being used by manufacturers to incorporate APIs into polymer systems.
In addition to APIs, novel drug-delivery systems are offering new ways to deliver medication to babies, toddlers, active youth, and middle- and advanced-age family members. Medication compliance is becoming easier to control and is having a beneficial impact on patient health.
The orthopedics industry has continually been improving the surface characteristics and properties of implant devices to give patients faster healing, reduced pain, and to ensure the long-term success of the devices. Antimicrobials have been used, and now, together with very effective bone-growth promoters, are giving the implants long-term survivability properties. Furthermore, the polymers of construction are giving the implants better functionality and improving their long-term use, preventing replacement procedures.
More-durable materials
Polymer manufacturers are continuing to improve their resins to bring better properties to target end-products. For instrument housings and medical durables, the materials of construction must be regularly cleaned and sanitized, often through exposure to aggressive cleaners and solvents. If not properly designed, the products and the materials from which they are made will deteriorate well before the end of their useful life. A manufacturer recently developed a nylon that can survive more than 500 autoclave cycles without clarity deterioration. EMS-Grivory America will describe the new material during a presentation at the Medical Design & Manufacturing (MD&M) West MiniTec event in Anaheim, CA, the week of Feb. 6, 2023. (The two-day MiniTec conference, sponsored by SPE, is scheduled for Feb. 6 and 7. MD&M West, which is co-located with Plastec West and three other events, runs from Feb. 7 to 9.)
|
Made of EMS Grilamid TR HT 200 developed at the EMS-Grivory R&D Center in Switzerland, this 2-mm plaque has undergone 500 cycles of autoclaving at 134°C. |
Similarly, a recently formed industry consortium will help establish common goals for performance, testing, and qualification of durable materials of construction used in healthcare environments. By having a unified voice, developers can strive for common characteristics and improve performance while reducing cost of development for all. This industry-led effort called HSI (HealthCare Surfaces Institute) is working to develop test standards that could allow OEMs to make and test their products before they enter the marketplace to determine their suitability for use.
Additive manufacturing makes its mark
Processing in 2023 will continue to support improved production and novel products. The rapid advancement of additive manufacturing (AM) systems has pushed industry to re-think how low-volume products should be made. AM parts have improved properties, excellent appearance, and can incorporate multiple materials. Changing from one size or feature to another is quick and low cost, allowing on-the-fly improvements of even production parts.
Micro-manufacturing capabilities are also expanding the frontiers of small part production with amazing functionality. Micro-produced parts are supporting the sophisticated diagnostics mentioned earlier, allowing better testing with minimal blood, saliva, or other samples. The smaller parts reduce manufacturing costs, give faster results, and help improve healthcare where used.
Harmonization finally within reach?
Material qualification almost uniformly relies on ISO test standards. This “harmonization” was recently seen when Swiss authorities introduced a measure that would allow products reviewed and cleared for use by US FDA to be cleared for use in their country. The adoption of the recently enacted Medical Device Regulation (MDR) has severely limited what is available for their citizenry. If Swiss legislators give full approval to the proposal, many products not currently available for use, if approved by FDA, could then be used in Switzerland.
I have long urged the harmonization of testing and regulations for medical devices by regulatory bodies of various governments. This proposal by Switzerland, if adopted, could be a model for other countries around the world.
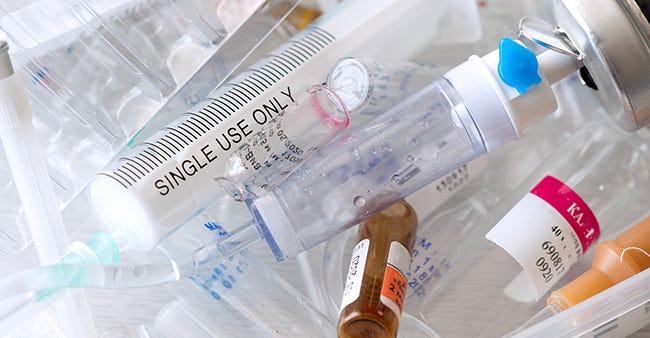
Circularity and the single-use device
Finally, as the world and the medtech community adjust to understanding that end-of-life issues for plastic products must be addressed by OEMs as well as product users, I believe that there is much to be done. To date, very little is being done to address the issue of what to do with single-use devices (SUDs) after their use. Virtually every medtech company has adopted the policy that no regrind is to be used in their products. This is to ensure that the materials of construction consistently meet all the required physical, chemical, and biological requirements, which are reviewed by FDA prior to market clearance for a product.
|
More needs to be done to address the issue of single-use medical devices in the waste stream. |
If systems can be developed to sort, decontaminate, and clean the SUDs after use, this waste stream can become part of the raw materials that go into the next batch of new products being made. Testing, of course, will need to be done to confirm suitability for use.
I believe that moving toward this goal of using more feedstock from used SUDs will require the cooperation of both FDA and medical device OEMs and the establishment of support structures not yet in place such as collection systems, sorters, cleaners, and processors that can prepare the once-used medical devices to become feedstock for new products. For those that say it can’t be done, I say look to the beverage bottle industry and check out the way PET bottles are reclaimed, ground, cleaned, and pelletized, making that material available for production of new PET beverage bottles. FDA has cleared these reprocessed materials for use in new beverage bottles. So, if it's acceptable for the beverage industry, we in the medical device industry should be able to qualify appropriately sorted and selected materials for reprocessing in medical devices.
In summary, I look forward to significant change in 2023 as new materials, processes, products, and procedures are introduced. The last three years of growth have generally been kept in the labs and manufacturing plants but are now ready for introduction to the user community. I urge everyone who can justify it, to get out and attend trade shows, conferences, and expositions. Nothing beats face-to-face engagement and witnessing first-hand the latest and greatest new offerings. My favorite is Medical Design & Manufacturing (MD&M) West in Anaheim, CA, the week of Feb. 7 to 9, 2023. I will be there, and I hope to see you there, as well!
About the author
|
Len Czuba is president of Czuba Enterprises Inc., a Chicago-based medical device product development consultancy. He works with clients to take products, especially medical devices, from concept to production. His primary focus is in the selection and processing of plastics and biomedical polymers used in medical devices.
Czuba holds 15 US patents, including several for PVC replacement materials. In 2004, he was named one of the 100 MD&DI Notable Persons in the medical device industry. He is a frequent conference organizer, speaker, and instructor and has given presentations and seminars around the globe.
A member of the Society of Plastics Engineers since 1975, Czuba is past chairman of the Medical Plastics Division, was Councilor for the European Medical Polymers Division, and was the society’s International President during SPE’s 2005/2006 year. He is now a Distinguished, Honored Service, Fellow of SPE.
You May Also Like