Medical molding applications, materials featured at Fakuma 2015
Fakuma returns to Friedrichshafen, Germany, next month for what is shaping up to be another record-breaking year. The show has been sold out since February 2015, and attendees will have an opportunity to witness advances in molding, extrusion, thermoforming, blowmolding and other plastics processing technologies along with innovations in materials and 3D printing.
September 8, 2015

Exhibitors often highlight medical applications at their booths to illustrate their achievements in precision and tight-tolerance production methods and compliance with rigorous quality standards. In this article, we preview some of those applications.
Fakuma 2015 will be held at the Friedrichshafen Messe from October 13 to 17. For more information about the event, go to the Fakuma website.
Mediprene introduces medical-grade TPEs
New Mediprene TPE compound grades for plunger seals in single-use syringes will be showcased by Elasto Sweden (Amal), part of the Hexpol TPE group, at booth B1 1217. The company has expanded its range of materials to meet a rise in demand for thermoplastic elastomers as a replacement for rubber, says Niklas Ottosson, Medical Technical Manager at Elasto.
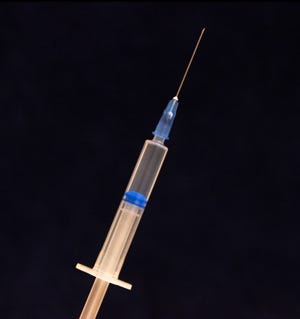 Mediprene grades withstand gamma, EtO and steam sterilization, and representative grades have passed cytotoxicity tests according to ISO 10993-5 and are compliant with USP Class VI biocompatibility guidelines. The latex-free materials are resistant to chemicals and fluids that are typically present in healthcare environments.
Mediprene grades withstand gamma, EtO and steam sterilization, and representative grades have passed cytotoxicity tests according to ISO 10993-5 and are compliant with USP Class VI biocompatibility guidelines. The latex-free materials are resistant to chemicals and fluids that are typically present in healthcare environments.
Mediprene grades are available in hardnesses ranging from 43 to 73 Shore A. The TPE seal mounted on the end of the plunger needs to provide a leakproof seal with the syringe barrel, and the low compression set of Mediprene TPEs helps to maintain seal integrity, says Elasto.
The medical-grade materials are available in translucent or colored compounds. Elasto reports that it has exercised extreme diligence in selecting the color masterbatch supplier to ensure that the pigments and carriers are compliant and that the masterbatches are manufactured under rigorous traceability, consistency and change-control procedures.
Processed at Elasto's ISO 13485–certified facilities, Mediprene TPEs feature cost and manufacturing advantages compared with thermoset rubber. They require no vulcanization and are 100% recyclable. Consistent shrinkage allows for tight dimensional tolerances with fewer process steps and without the need for additional operations, such as trimming.
KraussMaffei showcases molding of liquid-silicone rubber device
The physical and chemical properties of liquid silicone rubber (LSR) and high-temperature vulcanizing solid silicone are increasingly attracting the attention of medical device OEMs, notes KraussMaffei (Munich), which will demo the production of a nasal ventilator made of LSR at booth 7303 in hall 7.
The supplier of injection molding systems will run a four-piece mold on an all-electric AX with a 500-kN clamping force. An SPX 10 sprue picker will grip, separate and transfer the parts. The Elmet metering and mixing unit provides for optimal draining of residual fluids, ensuring that no material goes to waste.
The AX SilcoSet is suitable for use in cleanrooms and is approved for medical use.
All-electric AX series injection molding machines from KraussMaffei feature resource-efficient manufacturing with high repeatability. They earn a rating of Class 9+ on energy-efficiency tests.
Engel produces safety syringes using Fostag mold
 In the medical section of booth 5204 in hall A5, Engel (Schwertberg, Austria) will mold needle holders for 1-ml safety syringes using an e-motion 170/80 TL machine running a 16-cavity mold from Fostag Formenbau (Stein am Rhein, Switzerland). The filigree polystyrene parts, with a shot weight of 0.08 g, are designed with a predetermined breaking point that makes it impossible to re-use the disposable syringes.
In the medical section of booth 5204 in hall A5, Engel (Schwertberg, Austria) will mold needle holders for 1-ml safety syringes using an e-motion 170/80 TL machine running a 16-cavity mold from Fostag Formenbau (Stein am Rhein, Switzerland). The filigree polystyrene parts, with a shot weight of 0.08 g, are designed with a predetermined breaking point that makes it impossible to re-use the disposable syringes.
Thin, varying wall thicknesses require extremely precise process control. A clamping force that is too high or fluctuations in the melt volume immediately lead to rejects. To prevent this, two software solutions from Engel's iQ product family are used: iQ weight control automatically detects fluctuations in the melt volume and material viscosity and compensates for them in the same shot, while iQ clamp control continuously adjusts the clamping force to match process parameters based on mold breathing.
Arburg molds medical connectors
At booth 3101 in hall A3, Arburg (Lossburg, Germany) will display the electric Allrounder 470 A with a clamping force of 1,000 kN producing y connectors for an infusion therapy device using an eight-cavity mold from Männer. Featuring a cycle time of approximately 15 seconds, highlights of this application, says Arburg, include side injection through a needle-type shut-off nozzle and three-sided demolding of the PMMA parts, which weigh 1.1 grams.
The exhibit will include an extended conveyor belt with a tunnel cover for docking with the cleanroom. Placing the machine and peripheral equipment outside the cleanroom optimizes cost-effective operation, according to Arburg. A clean-air module with ionization above the clamping unit ensures ISO Class 7 clean atmosphere during series production.
DuPont showcases history of material innovation
DuPont (Geneva) announces that it will take the long view of its signature Zytel nylon material at booth 4201 in hall B4. Since inventing nylon in 1935 and modifying it to become the first true engineering-grade polymer, DuPont notes that the material has been used in numerous applications, including healthcare.
The DuPont booth at Fakuma will reflect how this advanced material, coupled with DuPont's broad material portfolio and application development support in the form of Computer Aided Engineering (CAE), can continue to create new growth opportunities in a wide range of industries. In the healthcare sector, its Special Control (SC) and Premium Control (PC) grades are described as providing an advanced degree of testing, manufacturing control and regulatory support for medical and healthcare applications.
"DuPont works with customers throughout the product development cycle, continually advancing materials, supporting design and optimizing for processing. It is helping customers predict how our materials will perform from art to part," says Michel Renaud, Head of Design for Europe, Middle East and Africa.
About the Author(s)
You May Also Like




