Pharma Company Sees Clear Advantages in COC Syringe
The company opted to use cyclic olefin copolymer (COC) for its new syringe because it exhibits glass-like clarity but is break-resistant and lightweight.
February 1, 2022
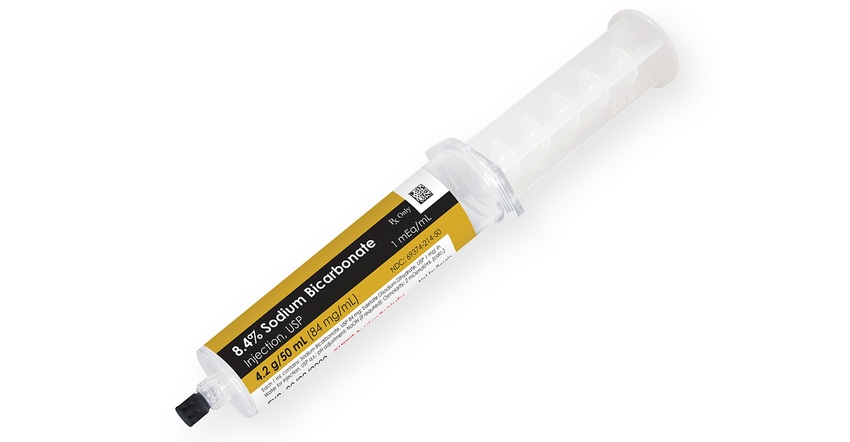
Nephron Pharmaceuticals Corp. has introduced a syringe made of cyclic olefin copolymer (COC) manufactured at its 503B outsourcing operation. COC features glass-like clarity but is break-resistant, lightweight, and withstands conventional sterilization methods. It is suitable for a range of medications, including viscous drugs, and provides for extended shelf life, said Nephron in making the announcement this week.
The first product set to launch in the new COC syringe is 8.4% sodium bicarbonate injection. Nephron said it plans to move more products to this format in the coming months.
"From lifesaving drugs to cutting-edge automation, Nephron is known for the innovative new ways it approaches pharmaceutical manufacturing," said Nephron CEO Lou Kennedy in a prepared statement. "That is why we could not be more excited or proud for the launch of our first COC syringe. In the same way we are transitioning products from traditional IV bags into blow-fill-seal plastic IV bottles, this product launch represents a progressive, and necessary, change, making life easier for healthcare professionals and products safer for patients."
For a deeper dive into the properties of cyclic olefin copolymers and the material's medical applications, read "Medical Plastics 101: Cyclic Olefin Copolymer Fulfills Complex Medtech Performance Requirements." |
Nephron’s 503B outsourcing facility division also produces pre-filled sterile syringes, luer-lock vials, IV bottles, and IV bags for US hospitals in an effort, the company claims, to alleviate drug-shortage needs.
Further catering to domestic healthcare gaps, Nephron also recently opened Nephron Nitrile, a plant that produces US-made, medical-grade nitrile gloves.
So-called 503B outsourcing facilities fall under section 503B of the Federal Food, Drug, and Cosmetic Act. Part of the Drug Quality and Security Act signed into law in November 2013, section 503B defines a compliant outsourcing facility as one that is engaged in the compounding of sterile drugs at one geographic location; is registered as an outsourcing facility; and meets all of the requirements of section 503B.
Based in West Columbia, SC, Nephron claims to be a world leader in the manufacture of generic respiratory medications. It has been certified as a woman-owned business by the National Women Business Owners Corp., and women make up nearly half of its workforce.
About the Author(s)
You May Also Like




