Replacing metal with plastics dramatically improves safety factor of surgical device
Transcatheter aortic valve replacement (TAVR) surgery, which is typically recommended for patients with severe aortic stenosis who are too ill to undergo open-heart surgery to replace a diseased aortic valve, can cause aortic regurgitation (AR). Also called aortic insufficiency, AR is a leaking of the heart's aortic valve that causes the blood to flow in the wrong direction, which can lead to serious complications.
September 17, 2015
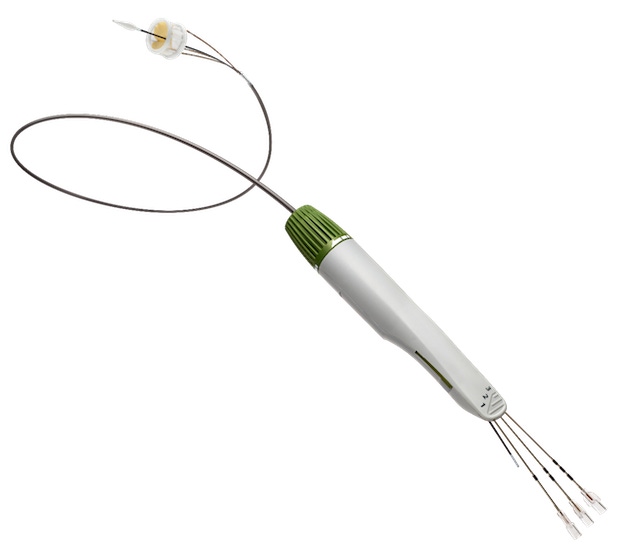
Transcatheter aortic valve replacement (TAVR) surgery, which is typically recommended for patients with severe aortic stenosis who are too ill to undergo open-heart surgery to replace a diseased aortic valve, can cause aortic regurgitation (AR). Also called aortic insufficiency, AR is a leaking of the heart's aortic valve that causes the blood to flow in the wrong direction, which can lead to serious complications. An all-plastic TAVR device developed by Direct Flow Medical (Santa Rosa, CA) may minimize the risk of AR, according to the company, because all of the device's metal parts were replaced with polymer-based materials. Sister brand Qmed spoke with the company's Director of Surgeon Relations, Randolph Chitwood, MD, who explained how material development enabled the design of "the gold standard" in surgical valves.
 Chitwood told Qmed that at the outset of the project the company was looking for a material with low viscosity that solidified quickly and was radiopaque. Moreover, it had to be "tolerable for patients" if it accidentally entered the bloodstream and had to be strong and creep resistant in its solid state. Unable to find an existing material that met all of those and other requirements, the company built a lab and assembled a team of device development engineers, material scientists and chemists with expertise in polymers.
Chitwood told Qmed that at the outset of the project the company was looking for a material with low viscosity that solidified quickly and was radiopaque. Moreover, it had to be "tolerable for patients" if it accidentally entered the bloodstream and had to be strong and creep resistant in its solid state. Unable to find an existing material that met all of those and other requirements, the company built a lab and assembled a team of device development engineers, material scientists and chemists with expertise in polymers.
"We looked at countless different chemical families and settled on a water-soluble epoxy backbone with an organically bound iodine additive for radiopacity," Chitwood told Qmed. "The real discovery came in finding the right amine blend. This is where . . . we were able to develop something that met all those divergent characteristics."
The polymer frame of the TAVR device is 30 times safer than conventional metal-based frames, contends Chitwood, who adds that this "revolutionary platform" is the "first and only commercial iteration of a polymer TAVR device."
The CE-marked product has been available in Europe since 2013 and is currently in clinical trials in the United States.
Read the full interview with Chitwood at Qmed.com.
Chitwood is scheduled to speak about the benefits of the all-plastic device for TAVR procedures at the medical manufacturing trade show and conference MD&M Philadelphia, which is co-located with PLASTEC Philadelphia, on Oct. 7 and 8.
About the Author(s)
You May Also Like




