Reva Medical accelerates development of thin-strut bioresorbable plastic stents
Reva Medical (San Diego, CA) is fast-tracking development of its Fantom family of thin-strut bioresorbable scaffolds made from a proprietary desaminotyrosine polycarbonate polymer. Fantom scaffolds reportedly achieve a 50% reduction in thickness compared with the company's ReZolve platform without a decrease in radial strength. They also result in manufacturing efficiencies. As a result, Reva will discontinue funding ReZolve-related projects, other than to follow patients in the Restore CE Mark trial, and focus entirely on the Fantom project.
March 27, 2014
Reva Medical (San Diego, CA) is fast-tracking development of its Fantom family of thin-strut bioresorbable scaffolds made from a proprietary desaminotyrosine polycarbonate polymer. Fantom scaffolds reportedly achieve a 50% reduction in thickness compared with the company's ReZolve platform without a decrease in radial strength. They also result in manufacturing efficiencies. As a result, Reva will discontinue funding ReZolve-related projects, other than to follow patients in the Restore CE Mark trial, and focus entirely on the Fantom project.
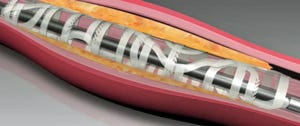 The thinner scaffold dimensions of the Fantom line improve deliverability and healing response, which can help to ensure broader adoption of the device. Fantom scaffolds also feature x-ray visibility and a single inflation to expand the device, attributes inherited from other Reva products that competitive polymer scaffolds do not offer, according to the company.
The thinner scaffold dimensions of the Fantom line improve deliverability and healing response, which can help to ensure broader adoption of the device. Fantom scaffolds also feature x-ray visibility and a single inflation to expand the device, attributes inherited from other Reva products that competitive polymer scaffolds do not offer, according to the company.
The first human clinical trials of the Fantom scaffold are expected by the end of 2014, with commercialization targeted for mid-2016.
"We are witnessing the conversion in an annual $4 billion market from metallic stents to the use of fully bioresorbable scaffolds, because these new devices help to restore blood flow in diseased heart vessels, then disappear when their jobs are complete," Reva CEO Bob Stockman is quoted as saying in a press release. "The coronary stent market is now demanding that bioresorbable scaffolds emulate the deliverability, scaffolding mechanics, and ease of use of today's best metallic stents. We believe that our new thin-strut technology family of Fantom scaffolds will best address these requirements," added Stockman.
PlasticsToday has followed the progress of Reva Medical's bioresorbable scaffold technology over the years. To learn more about the company's evolution, read "Reva Medical ramps up production of bioresorbable scaffold" and "Bioresorbable stent passes clinical tests in Brazil."
About the Author(s)
You May Also Like




