‘Diamonds from the sky’ approach grows carbon fiber from thin air
It may sound like something straight from Harry Potter, but the team at George Washington University that has found a way to economically convert atmospheric CO2 directly into highly valued carbon nanofibers is made up of real-life scientists, not wizards. The team presented their research on their new CO2 capture and utilization technology at the 250th National Meeting & Exposition of the American Chemical Society (ACS) last week in Boston.
August 28, 2015
It may sound like something straight from Harry Potter, but the team at George Washington University that has found a way to economically convert atmospheric CO2 directly into highly valued carbon nanofibers is made up of real-life scientists, not wizards. The team presented their research on their new CO2 capture and utilization technology at the 250th National Meeting & Exposition of the American Chemical Society (ACS) last week in Boston.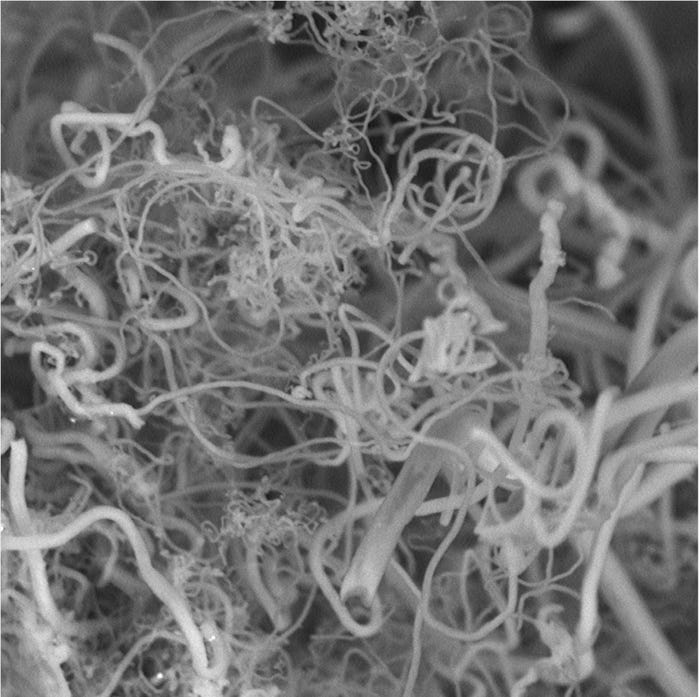 “We must find a way to mitigate the greenhouse gas CO2,†began Stuart Licht, Ph.D, who leads a research team at George Washington University. “Sequestration, which attempts to bind CO2 with other substances, is fraught with uncertainty, high costs and is unlikely to succeed.†His team has now come up with what he called “a viable solution to climate change." Their research could shift CO2 from a global-warming problem to a feedstock for the manufacture of in-demand carbon nanofibers.
“We must find a way to mitigate the greenhouse gas CO2,†began Stuart Licht, Ph.D, who leads a research team at George Washington University. “Sequestration, which attempts to bind CO2 with other substances, is fraught with uncertainty, high costs and is unlikely to succeed.†His team has now come up with what he called “a viable solution to climate change." Their research could shift CO2 from a global-warming problem to a feedstock for the manufacture of in-demand carbon nanofibers.
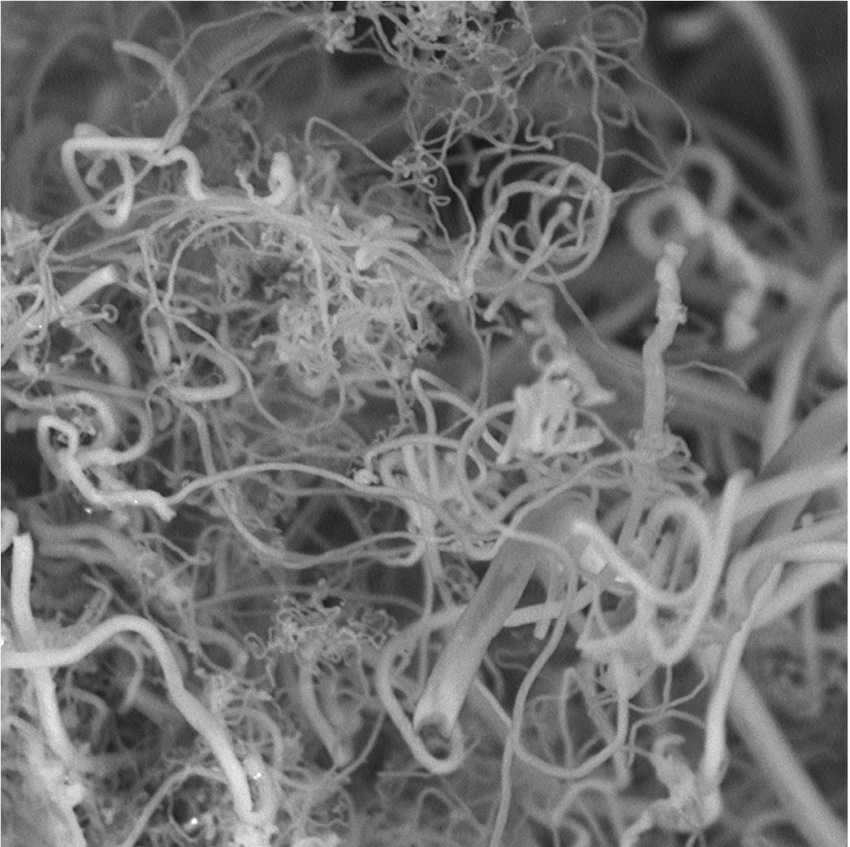
“We have found a way to use atmospheric CO2 to produce high-yield carbon nanofibers,†he said. “Such nanofibers are used to make strong carbon composites, such as those used in the Boeing Dreamliner, as well as in high-end sports equipment, wind turbine blades and a host of other products.â€
Licht calls his approach “diamonds from the sky.†That refers to carbon being the material that diamonds are made of, and also hints at the high value of the products, such as the carbon nanofibers that can be made from atmospheric carbon and oxygen.
According to Licht, the process is “very straightforward, simple chemistry,†which takes place in an electro-chemical reactor powered by solar energy. Because of its efficiency, this low-energy process can be run using only a few volts of electricity, sunlight and a whole lot of carbon dioxide. At its root, the system uses electrolytic syntheses to make the nanofibers. CO2 is broken down in a high-temperature electrolytic bath of molten carbonates at 1,380 degrees F (750 degrees C). Atmospheric air is added to an electrolytic cell. Once there, the CO2 dissolves when subjected to the heat and direct current through electrodes of nickel and steel. The carbon nanofibers build up on the steel electrode, where they can be removed.
Licht estimates electrical energy costs of this “solar thermal electrochemical process†to be around $1,000 per ton of carbon nanofiber product, which means the cost of running the system is hundreds of times less than the value of product output. “Carbon fiber is a very valuable product,†he pointed out. “Coal costs about $40 a ton, graphite is worth about $1,000 a ton; carbon fibers are worth on the order of about $25,000 a ton. We think this gives very large impetus to convert the carbon dioxide directly into carbon nanofibers from the atmosphere and provides a reasonable path to bring down CO2 levels in the atmosphere.â€
He added: “We calculate that with a physical area less than 10% the size of the Sahara Desert, our process could remove enough CO2 to decrease atmospheric levels to those of the pre-industrial revolution within 10 years.â€
At this time, the system is experimental, and Licht’s biggest challenge will be to ramp up the process and gain experience to make consistently sized nanofibers. Questioned about the scaling up of the process, however, he remained very upbeat. “It’s a very simple process to scale,†he said. “We’ve already shown that we can scale it up more than a hundred-fold. Our first attempt at scaling up was going from around 0.1 grams an hour to more than 10 grams an hour. It scales up quite easily—and the entire process is quite low energy.†He quickly added, however, that he didn’t want to minimize the amount of resources that will be needed for chemical engineers to correctly scale up and to continue the process.
Licht sees the carbon fiber market as being in a similar situation to that of the plastics market was at the beginning of World War 2. â€We believe that the carbon nanofiber market is just at the beginning, about to take off.â€
This process has the potential to y bring down the price of carbon nanofibers substantially, driving the demand for them.
Licht: “There will be a wonderful variety of applications, from building materials, renewable energy sources to nano-electronics: and with that we provide a wonderful buffer to store CO2 in a stable compact form.â€
The team’s research has been funded primarily by the National Science Foundation.
About the Author(s)
You May Also Like


