Acrylic magnesium nanocomposite advances hydrogen as fuel source
To replace gasoline as a fuel, hydrogen would have to be safely and densely stored, and while not possible now it might be in the future thanks to a newly developed acrylic-magnesium nanocomposite. Scientists with the U.S. Department of Energy (DOE) Lawrence Berkeley National Laboratory have designed the new polymethyl methacrylate (PMMA) and magnesium compound as a pliable nanocomposite that can absorb and release hydrogen at modest temperatures without oxidizing the metal after cycling.
March 14, 2011
To replace gasoline as a fuel, hydrogen would have to be safely and densely stored, and while not possible now it might be in the future thanks to a newly developed acrylic-magnesium nanocomposite. Scientists with the U.S. Department of Energy (DOE) Lawrence Berkeley National Laboratory have designed the new polymethyl methacrylate (PMMA) and magnesium compound as a pliable nanocomposite that can absorb and release hydrogen at modest temperatures without oxidizing the metal after cycling. The researchers call this a major breakthrough in materials design for hydrogen storage, batteries, and fuel cells.
The researchers work is highlighted in a paper entitled, "Air-stable magnesium nanocomposites provide rapid and high-capacity
acrylic magnesium nanocomposite for storing hydrogen |
High-capacity magnesium nanocrystals are encapsulated in a gas-barrier acrylic matrix for a new, composite hydrogen-storage material. |
hydrogen storage without heavy metal catalysts." The research work appears in the journal Nature Materials.
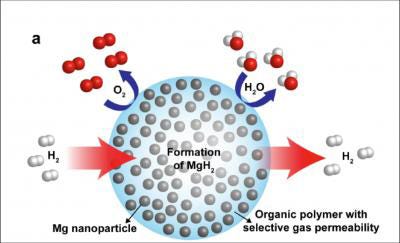
The Berkeley Lab scientists note in the paper that while there are a great variety of potential hydrogen sources, including water, biomass, and organic matter, the challenge that remains is producing a material that simultaneously absorbs hydrogen well enough to form a stable thermodynamic state, but does so weakly enough to release it on-demand with a small temperature rise.
The answer is an air-stable composite material that consists of metallic magnesium nanocrystals in the PMMA gas-barrier polymer matrix that enables both the storage of a high density of hydrogen (up to 6% by weight of magnesium and 4% by weight for the composite), as well as rapid kinetics, with loading in less than 30 minutes at 200°C. The researchers also note that nanostructuring of the magnesium provides rapid storage kinetics without using expensive heavy-metal catalysts.
The paper's authors included Jeff Urban, Ki-Joon Jeon, and Christian Kisielowski. The composite material was examined with the TEAM 0.5 electron microscope at the National Center for Electron Microscopy (NCEM), called the world's most powerful electron microscope.
About the Author(s)
You May Also Like