Sponsored By
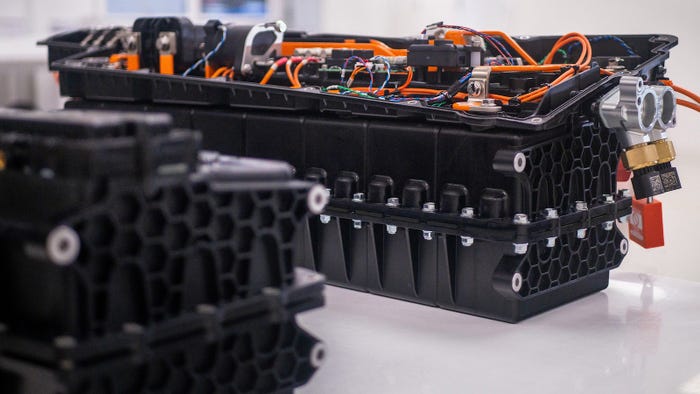
Celanese polyamide used in automotive parts
Automotive & Mobility
A Metal Replacement Star Is BornA Metal Replacement Star Is Born
Celanese introduced a polyamide reinforced with short glass fibers at Chinaplas this week that sets a new bar for lightweight, high-performance automotive materials.
Sign up for the PlasticsToday NewsFeed newsletter.